According to Data Bridge Market Research new Market report “Global Bioburden Testing Market, By Test Type (Aerobic, Anaerobic, Fungi, Spore), By Product Type (Instruments (PCR, Automated Microbial Identification Systems, Microscopes, Others), Consumables (Kits & Strips, Reagents, Others), By Technology (Microbial Filter Method, Advanced Colorimetric Method, Plate Count Method, Rapid Testing), By Application (Raw Material Testing, In-Process Testing, Medical Device Testing, Pharmaceutical & Cosmetic Testing, Food and Beverage Testing), By End-User (Pharmaceutical, Biotechnology, Industrial, Food & Beverages), By Geography (North America, Europe, Asia-Pacific, South America and Middle East Asia) – Industry Trends and Forecast to 2024”, is projected to reach USD 907.4 billion by 2024, at a CAGR of 9.1% during the forecast period from 2017 to 2024. Report Access: https://databridgemarketresearch.com/reports/bioburden-testing-market-industry-trends-and-forecast-to-2024/ Varying trends in the pharmaceutical and biotechnology industries, increasing medical device usage, market awareness and trend towards food safety, strong R&D investments in life science are the major factors contributing to the growth of global bioburden testing market. These technological advantage and cost effectiveness are the main driving factor for bioburden testing market in the forecast period 2017 to 2024. On the other hand, the towering cost instruments and compound time consuming rigid standards which extend the approval process in some counties may delay the growth of this market. Emerging economies such as India and China present a range of opportunities for this market. Companies into bioburden testing market are adopting popular strategies approaches such as innovative product launches, acquisition and others in order of expansion of market.
Market Segmentation: Bioburden Testing Market
- The Global Bioburden Testing Market is segmented on the basis of test type, product type, technology, application, end-user, and geography.
- On the basis of test type, the bioburden testing market is segmented into aerobic, anaerobic, fungi and spore count.
- The bioburden testing market segment is further categorized on the basis of product type into instruments and consumable.
- The market is also segmented on the basis of technology microbial filter method, advanced colorimetric method and plate count method. The plate count method and membrane filtration method can be used to analyze the number of microbes in sample.The sample is passing through a filter with a pore size of 0.45 micrometers or less. Oldest methods for bioburden testing plate count method, however is not used frequently on a great scale commercial basis due to its time consuming procedure. Advanced colorimetric method is at the moment the most capable and widely used bioburden testing technology. The gathering of colorimeter and computational capabilities has enabled advanced colorimetric methods to deliver swift results of quantifying bioburden.
Geographical Segmentation: Bioburden Testing Market
On the basis of geography the market is segmented into 5 geographical regions, North America, South America, Europe, Asia-Pacific and Middle East and Africa. The regions are further segmented into 15 chief countries such as US, Mexico, Canada, India, China, Japan, Korea, Southeast Asia, and Brazil. In 2017, North America is expected to dominate the bioburden testing market with 57.6% market share followed by Europe and APAC. However the APAC region is expected to grow at the highest CAGR of 17.4% in the forecast period 2017 to 2024. 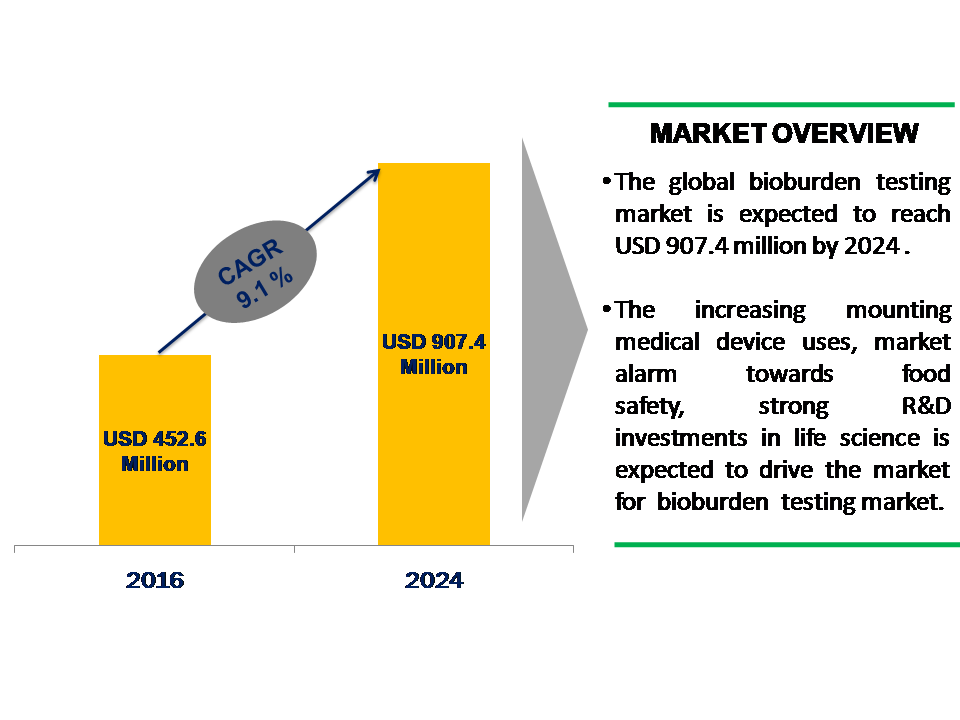
Competitive Landscape: Bioburden Testing Market
The global bioburden testing market report contains an in-depth profiling of the key market players, along with the recent developments (innovative product launches, agreements, joint ventures, business and alliance) and strategies adopted by them to continue and build up their positions in the market. Some of the major players operating in this market are Charles River Laboratories International, Inc., Pacific Bio labs, Inc., Sigma-Aldrich Corporation, SGS S.A., WuXi PharmaTech Inc., Becton, Dickinson and Company, Merck & Co., Inc., North American Science Associates, Inc. (NAMSA), Nelson Laboratories, Inc., Dynatec Labs, and ATS Labs, Inc. among others. Browse Related Reports: APAC Point-of-care Testing (POCT) Market Trends and Forecast to 2024 – By Product (Blood Glucose Testing Kits, Cardiometabolic Monitoring Kits, Infectious Disease Testing Kits, Cholesterol Testing Kits, Pregnancy & Fertility Tests Kits, Tumor/Cancer Markers, Urinalysis Testing Kits, Cholesterol Test Strips, Hematology Testing Kits, Drugs Of Abuse Testing Kits, Fecal Occult Testing Kits, Rapid Coagulation Testing Kits, & Others),By Prescription Mode (Prescription Based Testing & Over-The-Counter Testing),By Distribution Channel (Direct Tenders & Retail),By End User (Hospital, Clinics, Ambulatory Care, Home Healthcare, & Research Laboratory), By Country (China, Japan, India, South Korea, Australia, Thailand, Malaysia, Singapore, Indonesia, Philippines and Rest of APAC) https://databridgemarketresearch.com/reports/europe-radio-immunoassay-ria-reagents-and-devices-market Contact: Data Bridge Market Research Office Number 317, Amanora Chambers, Magarpatta Road, Hadapsar Pune – 411028 Maharashtra, India Toll Free: +1-888-387-2818 Mail:sales@databridgemarketresearch.com














