Global Urology Devices Market
Market Size in USD Billion
CAGR :
% 
 USD
42.87 Billion
USD
59.12 Billion
2024
2032
USD
42.87 Billion
USD
59.12 Billion
2024
2032
| 2025 –2032 | |
| USD 42.87 Billion | |
| USD 59.12 Billion | |
|
|
|
|
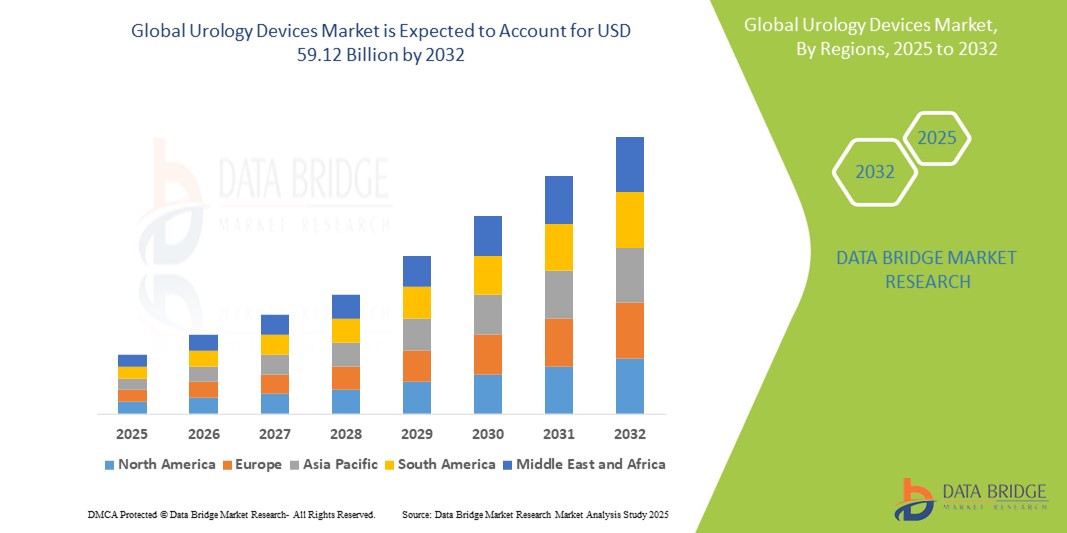
Urology Devices Market Size
- The global urology devices market size was valued at USD 42.87 billion in 2024 and is expected to reach USD 59.12 billion by 2032, at a CAGR of 4.10 % during the forecast period
- This growth is driven by factors such as the rising prevalence of urological disorders, increasing geriatric population, and advancements in minimally invasive surgical technologies
Urology Devices Market Analysis
- Urology devices are essential tools used in the diagnosis and treatment of urinary tract diseases and conditions such as urinary incontinence, kidney stones, and prostate disorders These devices include endoscopes, lasers, dialysis equipment, and urinary catheters
- The demand for urology devices is significantly driven by the rising geriatric population, increasing incidence of urological disorders, and growing preference for minimally invasive procedures
- North America is expected to dominate the urology devices market with a market share of 34.5%, due to advanced healthcare infrastructure, high adoption of cutting-edge medical technologies, and a strong presence of leading market players
- Asia-Pacific is expected to be the fastest growing region in the urology devices market with a market share of 26.5%, during the forecast period due to rapid advancements in healthcare infrastructure, rising awareness about urological health, and increasing volumes of urological surgeries
- Kidney Diseases segment is expected to dominate the market with a market share of 37.5% due to its high global prevalence, especially chronic kidney disease (CKD) and end-stage renal disease (ESRD)
Report Scope and Urology Devices Market Segmentation
|
Attributes |
Urology Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Urology Devices Market Trends
“Technological Innovations in Minimally Invasive and Robotic-Assisted Urology Procedures”
- One prominent trend in the urology devices market is the increasing adoption of minimally invasive and robotic-assisted technologies for urological procedures such as prostatectomy, nephrectomy, and lithotripsy
- These innovations are enhancing procedural efficiency, reducing patient recovery time, and improving surgical accuracy through greater precision and control
- For instance, robotic-assisted surgical systems enable 3D visualization and fine motor control, allowing urologists to perform complex procedures with minimal invasiveness, which is particularly impactful in treating prostate and kidney conditions
- These advancements are revolutionizing urological care, improving clinical outcomes, and driving the demand for technologically advanced urology devices across global healthcare systems
Urology Devices Market Dynamics
Driver
“Rising Incidence of Urological Disorders and Aging Population”
- The increasing prevalence of urological disorders such as benign prostatic hyperplasia (BPH), urinary incontinence, kidney stones, and prostate cancer is a major driver of demand for urology devices
- With the global population aging rapidly, age-related urological conditions are becoming more common, leading to a higher need for diagnostic and surgical interventions using advanced urology devices
- The surge in chronic conditions such as diabetes and hypertension also contribute to kidney-related complications, further fueling market growth for dialysis equipment and other urological tools
For instance,
- According to the Global Burden of Disease Study published by The Lancet in 2020, kidney disease was the 10th leading cause of death globally, with over 1.3 million deaths, highlighting the growing burden of urological conditions
- As these disorders become more prevalent, especially among the elderly, the demand for innovative and efficient urology devices continues to rise to improve patient outcomes and reduce surgical risks
Opportunity
“Integration of Artificial Intelligence in Urological Diagnostics and Surgery”
- The integration of artificial intelligence (AI) in urology is creating significant opportunities by enhancing diagnostic precision, predicting disease progression, and supporting robotic-assisted surgeries
- AI-driven platforms can analyze imaging data from ultrasound, CT, and MRI scans to detect urological conditions such as prostate cancer, kidney stones, and bladder abnormalities with improved accuracy and speed
- In surgical settings, AI can assist in real-time decision-making, optimize robotic system performance, and personalize treatment strategies by analyzing patient-specific data
For instance,
- In September 2024, according to a study published in The Journal of Urology, AI-based models demonstrated over 90% accuracy in detecting clinically significant prostate cancer through multiparametric MRI analysis, reducing the need for unnecessary biopsies and expediting treatment decisions
- The application of AI in urology is expected to streamline workflows, reduce diagnostic errors, and enhance patient outcomes, making it a transformative force in next-generation urological care
Restraint/Challenge
“High Equipment and Procedure Costs Limiting Accessibility”
- The high cost of advanced urology devices and associated surgical procedures remains a major restraint, particularly for healthcare facilities in low- and middle-income countries
- Urology equipment such as robotic surgical systems, laser lithotripsy devices, and endoscopic tools can range from tens of thousands to millions of dollars, creating financial strain for institutions with limited budgets
- These costs not only hinder procurement but also increase the overall expense of urological treatments, making them less accessible to economically disadvantaged populations
For instance,
- According to a 2023 report published by the International Society of Urology, the cost of robotic-assisted prostatectomy can be up to USD 15,000–USD 20,000 per procedure in the U.S., which poses affordability challenges for both providers and patients, especially where insurance coverage is limited or unavailable.
- Consequently, such financial barriers lead to disparities in care delivery, prevent timely adoption of innovative technologies, and ultimately restrict the growth potential of the global urology devices market, particularly in emerging region
Urology Devices Market Scope
The market is segmented on the basis of product, disease, technology, application, and end user.
|
Segmentation |
Sub-Segmentation |
|
By Product |
|
|
By Disease |
|
|
By Technology |
|
|
By Application |
|
|
By End User
|
|
In 2025, the kidney diseases is projected to dominate the market with a largest share in disease segment
The kidney diseases segment is expected to dominate the urology devices market with the largest share of 37.5% in 2025 due to its high global prevalence, especially chronic kidney disease (CKD) and end-stage renal disease (ESRD). The growing aging population, rising incidence of diabetes and hypertension, and increasing need for dialysis procedures further contribute to this dominance. Moreover, advancements in dialysis technologies and government initiatives for renal care are fueling segment growth
The dialysis devices is expected to account for the largest share during the forecast period in product type market
In 2025, the dialysis devices segment is expected to dominate the market with the largest market share of 35.8% due to its increasing global burden of chronic kidney disease (CKD) and end-stage renal disease (ESRD). The rising geriatric population, growing prevalence of diabetes and hypertension, and limited availability of kidney donors are driving demand for dialysis treatments. In addition, technological advancements and expanded access to dialysis services in emerging markets support this growth
Urology Devices Market Regional Analysis
“North America Holds the Largest Share in the Urology Devices Market”
- North America dominates the urology devices market with a market share of estimated 34.5%, driven, by advanced healthcare infrastructure, high adoption of cutting-edge medical technologies, and a strong presence of leading market players
- U.S. holds a market share of 30.5%, due to a high demand for advanced urology procedures, increasing prevalence of urological disorders such as prostate cancer, kidney diseases, and urinary incontinence, and continuous innovations in surgical technologies
- The availability of well-established reimbursement policies and a robust healthcare system further strengthen the market
- In addition, the increasing awareness about urological health and the adoption of minimally invasive and robotic-assisted surgeries are fueling market expansion in North America
“Asia-Pacific is Projected to Register the Highest CAGR in the Urology Devices Market”
- Asia-Pacific is expected to witness the highest growth rate in the urology devices market with a market share of 26.5%, driven by rapid advancements in healthcare infrastructure, rising awareness about urological health, and increasing volumes of urological surgeries
- Countries such as China, India, and Japan are emerging as key markets due to their growing aging populations, which are more susceptible to conditions such as BPH (benign prostatic hyperplasia), prostate cancer, and kidney diseases
- Japan, with its advanced medical technology and high adoption rates of minimally invasive and robotic-assisted surgical techniques, remains a crucial market for urology devices. The country continues to lead in adopting high-precision devices to improve surgical outcomes
- India is projected to register the highest CAGR with 4.6% market share in the global urology devices market, driven by expanding healthcare infrastructure, increasing prevalence of urological diseases, and rising investments in the adoption of advanced diagnostic and surgical technologies
Urology Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Medtronic (Ireland)
- Siemens (Germany)
- Abbott (U.S.)
- GE Healthcare (U.S.)
- BD (U.S.)
- Stryker (U.S.)
- Boston Scientific Corporation (U.S.)
- Cardinal Health (U.S.)
- Intuitive Surgical Operations, Inc. (U.S.)
- Cook (U.S.)
- Olympus Corporation (Japan)
- Johnson & Johnson Services, Inc. (U.S.)
- Fresenius Medical Care AG (Germany)
- Baxter (U.S.)
- Richard Wolf GmbH (Germany)
- Dornier MedTech (Germany)
- KARL STORZ SE & Co. KG (Germany)
- Endo, Inc. (U.S.)
- HealthTronics Inc. (U.S.)
- MEDI TECH DEVICES PVT LTD (India)
- Coloplast A/S (U.S.)
Latest Developments in Global Urology Devices Market
- In March 2025, Olympus Corporation introduced an AI-powered surgical planning tool designed to enhance imaging and machine learning capabilities in diagnosing urological diseases. This tool assists in minimally invasive nephrectomies by accurately assessing the size, location, and shape of tumors, cysts, or other abnormalities, thereby improving surgical precision and patient outcomes
- In April, 2023, Boston Scientific Corp. reported a significant surge in its stock value, attributed to approximately 90 new product launches, including advancements in urology devices. The company's strong performance and optimistic guidance reflect its continued investment in expanding its urology device portfolio
- In December 2023, Cook Medical received premarket approval from the U.S. Food and Drug Administration (FDA) for its Zenith Alpha Thoracic Endovascular Graft. This device is indicated for the endovascular treatment of patients with isolated lesions of the descending thoracic aorta. The Zenith Alpha Thoracic Graft features a lower-profile delivery system, enhancing its suitability for patients with smaller iliac arteries and facilitating easier deployment in tortuous anatomy
- In May 2021, Olympus Corporation introduced the Soltive SuperPulsed Laser System, a next-generation laser technology for urological procedures. This system utilizes superpulsed laser technology to provide enhanced precision and control during treatments for kidney stones and benign prostatic hyperplasia (BPH). The Soltive Laser System offers improved tissue interaction and reduced thermal damage, contributing to better patient outcomes and shorter recovery times
- In May 2021, Teleflex announced that its UroLift Advanced Tissue Control (ATC) System received clearance from the U.S. Food and Drug Administration (FDA). The UroLift ATC System is designed to treat lower urinary tract symptoms due to benign prostatic hyperplasia (BPH) by providing improved tissue manipulation and control during the procedure. This advancement aims to enhance the efficiency and effectiveness of the UroLift System, offering patients a minimally invasive treatment option for BPH
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.