Europe In Situ Hybridization Devices Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
160.00 Million
USD
206.56 Million
2024
2032
USD
160.00 Million
USD
206.56 Million
2024
2032
| 2025 –2032 | |
| USD 160.00 Million | |
| USD 206.56 Million | |
|
|
|
|
Europe In-Situ Hybridization Devices Market Segmentation, By Technology (Fluorescence in-Situ Hybridization (FISH), Chromogenic in Situ Hybridization (CISH)), Probe Type (DNA, RNA), Device (Instruments, Consumables & Accessories, Software, Services), Application (Cancer, Cytogenetics, Developmental Biology, Infectious Diseases, Others), End User (Research & Diagnostic Laboratories, Pharmaceutical & Biotechnology Companies, Academic Institutes, CROs, Others) - Industry Trends and Forecast to 2032
In-Situ Hybridization Devices Market Size
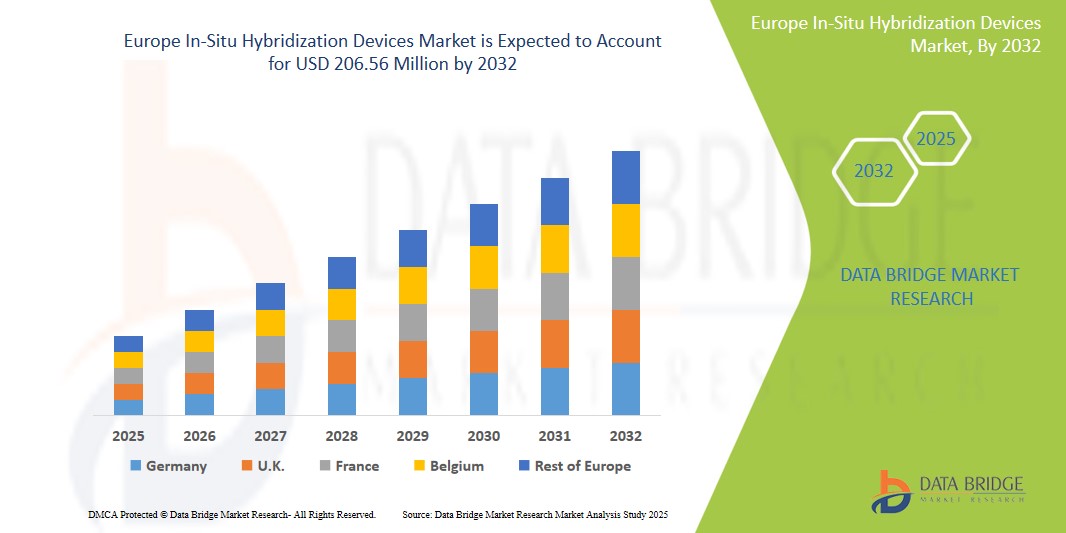
- The Europe In-Situ Hybridization Devices Market was valued atUSD160 million in 2024 and is expected to reachUSD206.56 million by 2032, at aCAGR of 3.2%During the forecast period.
- The Europe In-Situ Hybridization Devices Market is primarily driven by several key factors. These include the rising incidence of genetic disorders and cancer, which has increased the demand for advanced diagnostic techniques like in-situ hybridization (ISH), and the growing adoption of personalized medicine.
Europe In-Situ Hybridization Devices Market Analysis
- In-Situ Hybridization Devices play a crucial role in modern healthcare management across Europe. These solutions enable the precise detection of genetic material in tissues and cells, improving the accuracy of diagnoses for conditions like cancer, genetic disorders, and infectious diseases. Their use significantly contributes to the optimization of healthcare systems, facilitating early disease detection and targeted treatment, ultimately enhancing overall patient outcomes across the region.
- The demand for In-Situ Hybridization Devices in Europe is primarily driven by factors such as the increasing need for advanced diagnostic techniques, the growing prevalence of genetic diseases, and the rising demand for personalized medicine. Additionally, technological advancements in ISH platforms, including improved sensitivity and automation, are making these devices more accessible to a broader range of healthcare providers, thereby supporting market expansion.
- Europe holds a significant position in the global In-Situ Hybridization Devices market, supported by a robust healthcare infrastructure, a strong focus on research and development, and widespread adoption of cutting-edge diagnostic technologies. Countries like Germany, the UK, and France are leading the regional market due to their strong medical research foundations, high healthcare spending, and supportive government initiatives aimed at enhancing diagnostic capabilities.
- Regulatory support from European healthcare agencies, along with increasing investments in genomic research and personalized healthcare solutions, are further propelling the market. Moreover, collaborations between academic institutions, healthcare providers, and industry players are driving innovation in ISH technology, ensuring more effective diagnostics and treatments for various genetic and chronic conditions across Europe.
Report ScopeIn-Situ Hybridization DevicesMarket Segmentation
|
Attributes |
In-Situ Hybridization DevicesKey Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
In-Situ Hybridization Devices Market Trends
“Integration of Artificial Intelligence and Automation”
- One of the key trends in the Europe In-Situ Hybridization Devices Market is the integration of Artificial Intelligence (AI) and automation in hybridization techniques. AI-driven platforms are enabling more accurate and faster analysis of genomic data, reducing human error and increasing the throughput of genetic tests. AI is increasingly being used to interpret complex hybridization patterns, enhance image analysis, and provide predictive insights into genetic disorders. Automation in sample handling and image scanning has improved the consistency and reproducibility of test results, accelerating the diagnostic process.
- For instance, several European laboratories are adopting AI-based diagnostic tools in combination with In-Situ Hybridization Devices to streamline the analysis of molecular probes, thus improving efficiency in detecting genetic mutations and disorders.
- Another notable trend is the increasing use of personalized medicine driven by In-Situ Hybridization Devices. With the rise of precision medicine, these devices are playing an increasingly critical role in tailoring treatments based on individual genetic profiles. This trend is particularly important in oncology and rare genetic disorders, where specific biomarkers identified through hybridization techniques enable more effective and customized treatment regimens. With advancements in AI, the interpretation of these complex data sets is becoming more refined, leading to better patient outcomes across Europe.
In-Situ Hybridization Devices Market Dynamics
Driver
“Rising Demand for Personalized Medicine and Advancements in Genetic Research”
- One of the key drivers propelling the growth of the Europe In-Situ Hybridization Devices Market is the increasing demand for personalized medicine. As genetic research continues to progress, there is a growing need for precise diagnostic tools that can detect specific genetic variations. In-Situ Hybridization (ISH) devices, which allow for the visualization of specific DNA or RNA sequences, are crucial in this regard, supporting the move toward more tailored treatment plans.
- The growing emphasis on precision medicine, particularly in oncology and rare genetic disorders, is driving the adoption of advanced molecular diagnostic tools, such as ISH devices, to identify genetic mutations and provide targeted treatments. With the rise of personalized healthcare, these devices are becoming an integral part of diagnostic laboratories across Europe.
- As healthcare providers embrace these advancements, ISH devices help improve patient outcomes by enabling more accurate diagnoses and better-informed treatment decisions.
- For instance, The UK’s National Health Service (NHS) has supported genetic research and personalized medicine initiatives, which has increased demand for advanced diagnostic devices, including ISH.
- In Germany, precision medicine initiatives are fueling the adoption of ISH technology for the identification of genetic mutations and the development of tailored therapies.
Opportunity
“Integration of Artificial Intelligence (AI) for Enhanced Diagnostic Accuracy”
- A significant opportunity in the Europe In-Situ Hybridization Devices Market lies in the integration of AI-powered platforms to enhance diagnostic accuracy. AI-driven image analysis tools are being used to analyze complex hybridization patterns more efficiently, allowing for more accurate interpretation of genetic data. This improves the ability to detect genetic abnormalities, thus aiding in early diagnosis and personalized treatment.
- AI also accelerates the analysis of large volumes of patient data and supports real-time decision-making, which is particularly beneficial in high-throughput laboratories. The adoption of AI technology, when combined with ISH devices, enhances the capabilities of healthcare providers and labs to offer timely and precise genetic diagnoses.
- For instance, In France, AI-enhanced diagnostic platforms are being integrated into clinical laboratories, driving the demand for ISH devices and supporting improved diagnostic workflows.
- In Sweden, AI technologies are being used in research facilities to streamline data interpretation, making ISH devices more efficient in detecting genetic markers.
Restraint/Challenge
“High Costs and Technical Complexity of In-Situ Hybridization Devices”
- One significant challenge in the Europe In-Situ Hybridization Devices Market is the high cost and technical complexity associated with these devices. While ISH devices offer accurate and detailed genetic analysis, they require significant investment in both the equipment and skilled personnel to operate. The complexity of handling these devices and interpreting the results can be a barrier, particularly for smaller healthcare facilities or those in developing regions.
- Additionally, the maintenance and calibration of ISH devices require ongoing costs, and training staff to use the technology effectively can add further financial burdens. These factors could deter some healthcare providers from adopting ISH technology, especially in countries with limited healthcare budgets or in regions where funding for advanced medical technologies is constrained.
- For instance, In Eastern European countries, the high upfront costs and technical training required for ISH devices are delaying widespread adoption, limiting the growth potential in these regions.
- In Italy, certain public healthcare institutions are struggling with the financial costs of purchasing and maintaining advanced diagnostic equipment like ISH devices, which has hindered the rollout of such technologies in some areas.
In-Situ Hybridization Devices Market Scope
The market is segmented into four notable segments based on technology, device, application, and end user.
|
Segmentation |
Sub-Segmentation |
|
By Technology |
|
|
By device |
|
|
By Application |
|
|
By End User |
|
In 2025, the Fluorescence in-Situ Hybridization (FISH) is projected to dominate the market with a largest share in the technology segment
The Fluorescence in-Situ Hybridization (FISH) is expected to lead the Europe In-Situ Hybridization Devices Market with the largest share of 20.42% in 2025.This dominance is driven by its high sensitivity and specificity in detecting genetic abnormalities and chromosomal alterations, making it a preferred method for diagnosing genetic diseases, cancer, and other molecular disorders.
The Instruments is expected to account for the largest share during the forecast period in device market
In 2025, the Instruments segment is expected to dominate the market with the largest market share of 41.11% the increasing demand for advanced, high-precision tools that are essential for performing accurate in-situ hybridization procedures. These instruments, including hybridization ovens, microscopes, and staining systems, are crucial for ensuring high-quality results in molecular diagnostics and genetic research.
In-Situ Hybridization Devices Market Regional Analysis
“Germany is the Dominant Country in the In-Situ Hybridization Devices Market”
- Germany dominates the European In-Situ Hybridization Devices Market, accounting for the largest share due to its advanced healthcare infrastructure, high research and development expenditure, and a strong focus on integrating cutting-edge medical technologies.
- The country’s early adoption of in-situ hybridization techniques for genetic diagnostics and molecular research has significantly contributed to its market leadership.
- Major players, such as Siemens Healthineers and Biozym, are headquartered in Germany, providing a broad range of high-quality in-situ hybridization devices that comply with stringent European regulatory standards.
- Government initiatives to promote personalized medicine, genomics research, and investments in healthcare technology further strengthen Germany’s position in the European In-Situ Hybridization Devices Market.
“France is Projected to Register the Highest Growth Rate”
- France is expected to register the highest growth in the European In-Situ Hybridization Devices Market, driven by its expanding molecular diagnostic capabilities and rising adoption of advanced genetic testing methods.
- The country’s robust public healthcare system, coupled with increasing investments in genomics and personalized medicine, is fueling demand for more precise and efficient hybridization technologies.
- Government-backed initiatives aimed at enhancing digital healthcare infrastructure, alongside rising healthcare research investments, are accelerating the adoption of in-situ hybridization devices in France.
- Furthermore, the increasing use of in-situ hybridization in cancer research and diagnostics is further propelling market growth in France, with collaborations between public and private institutions driving technological advancements in this space.
In-Situ Hybridization Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, Europe presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Bayer AG (Germany)
- Merck & Co., Inc. (Germany/USA)
- Siemens Healthineers (Germany)
- SAP SE (Germany)
- Cerner Corporation (USA)
- Accenture (Ireland/USA)
- Philips Healthcare (Netherlands)
- IBM Corporation (USA)
- TietoEVRY (Finland)
- Optum (USA)
Latest Developments in Europe In-Situ Hybridization Devices Market
- In April 2021, Bio-Techne announced the commercial launch of its Novel DNAscope ISH assay for chromogenic detection of structural variations and DNA copy numbers. Unlike others, the commercially available assays, DNAScope enables high resolution and targeted detection of small genomic regions or single gene locus with its proprietary signal amplification system coupled with oligo probes.
- In February 2023, Molecular Instruments, a spin-off of the California Institute of Technology, launched HCR RNA-CISH kits to enhance automated chromogenic ISH workflows working on RNAscope. These kits provide double turnaround time and half the cost
- In January 2023, Ikonisys SA partnered with Integrated Gulf Biosystems Group (IGB) to distribute Ikoniscope20 Digital Fluorescence Microscope Solution in the Middle East, including Saudi Arabia, UAE, Kuwait, Bahrain, and South Asian markets. This collaboration aims to expand the reach of Ikonisys' oncology testing solutions
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.