Global Tardive Dyskinesia Treatment Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
324.82 Million
USD
452.12 Million
2024
2032
USD
324.82 Million
USD
452.12 Million
2024
2032
| 2025 –2032 | |
| USD 324.82 Million | |
| USD 452.12 Million | |
|
|
|
|
Global Tardive Dyskinesia Treatment Market Segmentation, By Treatment (Medication and Surgery), Drugs (Antipsychotics, VMAT2 Inhibitors, Other Neurological Agents, Botulinum Toxin and Natural Remedies), Route of Administration (Oral and Injectable), End- Users (Hospitals, Homecare, Specialty Clinics, and Others), Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Others) - Industry Trends and Forecast to 2032
Tardive Dyskinesia Treatment Market Size
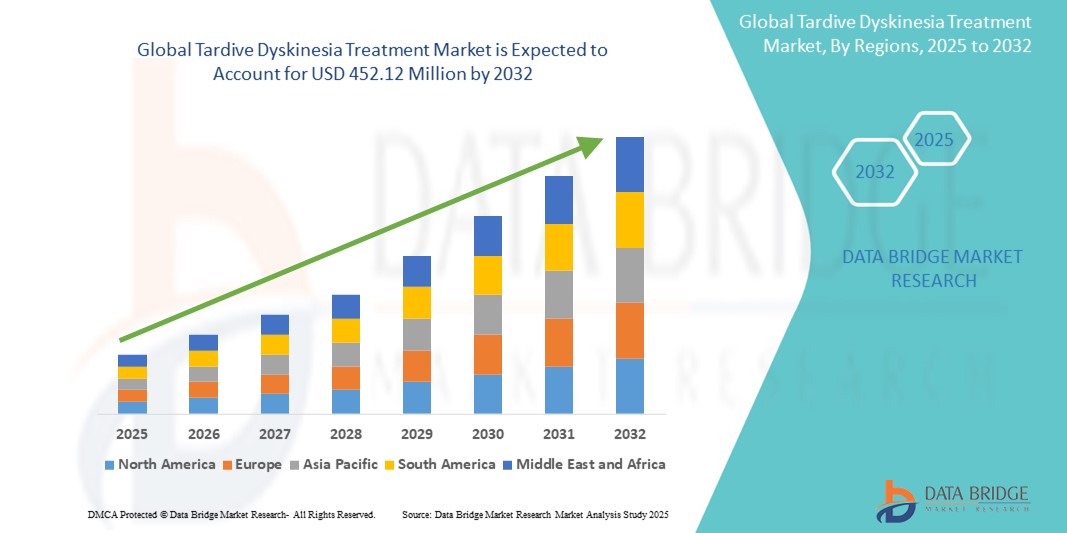
- The global tardive dyskinesia treatment market size was valued atUSD 324.82 million in 2024and is expected to reachUSD 452.12 million by 2032, at aCAGR of 4.22%during the forecast period
- This growth is driven by factors such as the rising prevalence of tardive dyskinesia, increasing awareness and diagnosis rates, and advancements in therapeutic options, including the approval of novelVMAT2 inhibit
Tardive Dyskinesia Treatment Market Analysis
- Tardive dyskinesia (TD) is aneurological disordercaused by long-term use of antipsychotic medications, characterized by involuntary, repetitive movements, primarily affecting the face and tongue
- The market growth is primarily driven by increasing prevalence of psychiatric disorders such asschizophreniaandbipolar disorder, along with rising awareness and early diagnosis of TD
- North America is expected to dominate the Tardive Dyskinesia Treatments market with a market share of estimated 45.05%,driven, by high prevalence of psychiatric disorders, advanced healthcare infrastructure, and strong availability of FDA-approved therapies such as valbenazine and deutetrabenazine
- Asia-Pacific is expected to be the fastest growing region in the tardive dyskinesia treatment market during the forecast period due to increasing mental health awareness, improving access to healthcare, and a growing patient population
- Vesicular Monoamine Transporter 2 (VMAT 2) segment is expected to dominate the market with a market share of 55.05% due to its proven efficacy of VMAT2 inhibitors, such as valbenazine and deutetrabenazine, in reducing symptoms of tardive dyskinesia. These drugs are specifically designed to target the underlying neurochemical imbalance, offering better symptom management and fewer side effects compared to traditional treatments
Report Scope and Tardive Dyskinesia Treatment Market Segmentation
|
Attributes |
Tardive Dyskinesia Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Tardive Dyskinesia Treatment Market Trends
“Shift Toward Targeted Therapies and Personalized Treatment Approaches”
- A key trend in the tardive dyskinesia treatment landscape is the growing shift toward targeted therapies, particularly VMAT2 inhibitors, which address the disorder's underlying neurochemical causes with greater specificity
- This trend reflects an increased focus on personalized medicine, where treatments are tailored based on individual patient profiles, improving therapeutic outcomes and minimizing adverse effects
- For instance, VMAT2 inhibitors such as valbenazine and deutetrabenazine are gaining prominence due to their favorable safety profile and efficacy in reducing involuntary movements, significantly enhancing quality of life for patients with tardive dyskinesia
- This transition toward precision therapies is reshaping the market, fostering innovation in drug development and expanding access to advanced, patient-centric treatment options
Tardive Dyskinesia Treatment Market Dynamics
Driver
“Rising Prevalence of Mental Health Disorders and Antipsychotic Use”
- The increasing global burden of psychiatric disorders such as schizophrenia, bipolar disorder, and major depressive disorder is a major factor driving the demand for tardive dyskinesia treatment
- These conditions are commonly managed with long-term antipsychotic medications, which significantly elevate the risk of developing tardive dyskinesia as a side effect
- As awareness grows and diagnosis rates improve, a larger patient pool is being identified and treated, boosting the demand for effective therapies that can manage or reverse the symptoms of tardive dyskinesia
For instance,
- According to the World Health Organization’s 2022 report, approximately 1 in 8 people globally live with a mental disorder, highlighting the scale of antipsychotic use and associated complications such as tardive dyskinesia
- This rising mental health burden and increased use of antipsychotics are directly contributing to the growth of the tardive dyskinesia treatment market, as healthcare systems prioritize safer and more effective long-term solutions
Opportunity
“Emerging Role of Digital Health and Telemedicine in TD Management”
- The integration of digital health technologies and telemedicine platforms presents a major opportunity for improving access to tardive dyskinesia (TD) diagnosis and treatment, especially in underserved and remote regions
- Telehealth solutions allow psychiatrists and neurologists to monitor patients remotely, track medication side effects in real time, and make timely adjustments to treatment plans, enhancing long-term care for TD patients
- Digital tools, including mobile health apps and wearable devices, can also support patient engagement and adherence by reminding users to take medications, log symptoms, and report side effects to their healthcare providers
For instance,
- In a 2024 study published in Frontiers in Psychiatry, telepsychiatry was shown to increase follow-up care adherence by 32% among patients with antipsychotic-induced movement disorders, demonstrating its potential to improve TD outcomes
- The growing adoption of telemedicine in mental health services enables early detection and management of tardive dyskinesia, reduces healthcare costs, and enhances patient quality of life by ensuring continuous and accessible care
Restraint/Challenge
“High Treatment Costs and Limited Access to Novel Therapies”
- The high cost of approved tardive dyskinesia treatments, particularly VMAT2 inhibitors, presents a significant barrier to widespread adoption, especially in low- and middle-income regions
- Medications such as valbenazine and deutetrabenazine can cost several thoU.S.nd dollars annually, making them unaffordable for many patients without comprehensive insurance coverage or public health support
- This cost-related barrier limits access to effective treatments, forcing some patients to rely on less effective or off-label therapies, which may not provide optimal relief or carry greater side effect risks
For instance,
- According to a 2023 cost analysis published by the Journal of Managed Care & Specialty Pharmacy, the average annual cost of VMAT2 inhibitors in the U.S. exceeds USD 90,000, highlighting the affordability challenge for uninsured and underinsured populations
- Consequently, the financial burden on healthcare systems and patients may impede market penetration, particularly in developing regions, and restrict the equitable distribution of advanced therapeutics
Tardive Dyskinesia Treatment Market Scope
The market is segmented on the basis of treatment, drugs, route of administration, end users, and distribution channel
|
Segmentation |
Sub-Segmentation |
|
By Treatment |
|
|
By Drugs |
|
|
By Route of Administration |
|
|
By End Users |
|
|
By Distribution Channel |
|
In 2025, the Vesicular Monoamine Transporter 2 (VMAT2) is projected to dominate the market with a largest share in drugs segment
The Vesicular Monoamine Transporter 2 (VMAT2) segment is expected to dominate the tardive dyskinesia treatment market with the largest share of 55.05% in 2025 due to its the proven efficacy of VMAT2 inhibitors, such as valbenazine and deutetrabenazine, in reducing symptoms of tardive dyskinesia. These drugs are specifically designed to target the underlying neurochemical imbalance, offering better symptom management and fewer side effects compared to traditional treatments. The increasing adoption of these FDA-approved drugs by healthcare providers and their widespread recognition in clinical practice further solidifies their market leadership
The botulinum toxin is expected to account for the largest share during the forecast period in drugs market
In 2025, the botulinum toxin segment is expected to dominate the market with the largest market share of 35.05% due to its effectiveness in managing focal and segmental dystonias associated with tardive dyskinesia. Its ability to provide targeted muscle relaxation with minimal systemic side effects makes it a preferred option, especially for patients unresponsive to oral medications. In addition, growing clinical evidence supporting its use and rising adoption in specialty clinics contribute to its expanding market presence
Tardive Dyskinesia Treatment Market Regional Analysis
“North America Holds the Largest Share in the Tardive Dyskinesia Treatment Market”
- North America dominates the tardive dyskinesia treatment market with a market share of estimated 45.05%, driven, by high prevalence of psychiatric disorders, advanced healthcare infrastructure, and strong availability of FDA-approved therapies such as valbenazine and deutetrabenazine
- U.S. holds a market share of 76.18%, due to high rates of antipsychotic prescriptions, increased awareness among healthcare providers, and favorable reimbursement policies for neurological and psychiatric treatments
- The presence of leading pharmaceutical companies investing in R&D for movement disorders, along with early adoption of innovative therapeutic approaches, further supports market growth
- In addition, the growing mental health initiatives and telemedicine services in the region contribute to increased diagnosis and timely management of tardive dyskinesia
“Asia-Pacific is Projected to Register the Highest CAGR in the Tardive Dyskinesia Treatment Market”
- Asia-Pacific is expected to witness the fastest growth in the tardive dyskinesia treatment market, driven by improving access to mental healthcare, increasing awareness of treatment options, and rising U.S.ge of antipsychotic medication
- Countries such as China, India, and Japan are seeing growing incidences of psychiatric disorders, spurred by urban stress, demographic shifts, and better diagnostic capabilities, leading to an expanding patient base
- Japan remains a key market due to its highly developed pharmaceutical sector and proactive adoption of specialized treatments for neurological conditions
- China and India are rapidly scaling up their mental health infrastructure and are increasingly targeted by pharmaceutical firms for clinical trials and commercial launches, supported by favorable government initiatives and expanding healthcare expenditure
Tardive Dyskinesia Treatment Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Neurocrine Biosciences, Inc.(U.S.)
- Teva Pharmaceutical Industries Ltd.(Israel)
- H. Lundbeck A/S(Denmark)
- AbbVie Inc.(U.S.)
- Otsuka Holdings Co., Ltd.(Japan)
- Astellas Pharma Inc. (Japan)
- Bausch Health Companies Inc. (Canada)
- Sumitomo Pharma America, Inc (U.S.)
- Reviva Pharmaceuticals Holdings, Inc. (U.S.)
- Acadia Pharmaceuticals Inc. (U.S.)
- VANDA PHARMACEUTICALS (U.S.)
- Eisai Co., Ltd. (Japan)
- Zydus Group (India)
- Lilly (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Pfizer Inc. (U.S.)
- Horizon Therapeutics (U.S.)
- Mundipharma (U.K.)
- Bristol-Myers Squibb Company (U.S.)
- Mallinckrodt (U.S.)
Latest Developments in Global Tardive Dyskinesia Treatment Market
- In July 2024, Neurocrine Biosciences introduced a new sprinkle formulation of INGREZZA (valbenazine) capsules, designed to ease administration for individuals experiencing dysphagia or difficulty swallowing. This formulation allows the contents to be sprinkled on soft foods, facilitating treatment adherence for adults with tardive dyskinesia (TD) and chorea associated with Huntington's disease. The sprinkle capsules are available in 40 mg, 60 mg, and 80 mg strengths
- In May 2024, During Tardive Dyskinesia Awareness Week, Neurocrine Biosciences collaborated with various stakeholders to conduct nationwide initiatives aimed at decreasing stigma, improving recognition, and increasing routine screenings and diagnosis of TD. These efforts are part of a broader commitment to enhance awareness and support for the approximately 600,000 individuals in the U.S. living with TD
- In April 2024, The U.S. Food and Drug Administration (FDA) approved the sprinkle formulation of INGREZZA (valbenazine) capsules for the treatment of adults with tardive dyskinesia and chorea associated with Huntington's disease. This approval provides a more convenient administration option for patients who have difficulty swallowing pills, potentially improving treatment adherence and outcomes
- In February 2023, Teva Pharmaceuticals, a U.S. affiliate of Teva Pharmaceutical Industries Ltd. announced that the U.S. Food and Drug Administration (FDA) has approved AUSTEDOXR (deutetrabenazine) extended-release tablets, a new once-daily formulation indicated in adults for tardive dyskinesia (TD) and chorea associated with Huntington’s disease (HD). This strategy enabled company to strengthen their product offerings
- In June 2022, Mitsubishi Tanabe Pharma Corporation a member of the Mitsubishi Chemical Holdings Group, announced that MTPC is launching DYSVAL capsules 40mg (the vesicular monoamine transporter type 2 (VMAT2) inhibitor) for the treatment of tardive dyskinesia in Japan. This strategy enabled company to expand its customer base
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.