North America Pancreatic Cancer Diagnostics Market, By Test Type (Imaging Test, Biopsy, Blood Test, Genomic Test, and Others), Cancer Stage (Stage 0, Stage I, Stage II, Stage III and Stage IV), Tumor Type (Exocrine Tumors and Neuroendocrine Tumors), Product (Instrument-Based Products, Platform-Based Products, Kits and Reagents, and Other Consumables), Technology (Fluorescent In Situ Hybridization, Next Generation Sequencing, Fluoroimmunoassay, Comparative Genomic Hybridization, Immunohistochemical, and Others), Application (Screening, Diagnostic and Predictive, Prognostic, and Research), End User (Hospitals, Diagnostic Centers, Cancer Research Centers, Academic Institutes, Ambulatory Surgical Centers, and Others), Distribution Channel (Direct Tender, Retail Sales and Others), Industry Trends and Forecast to 2030.
North America Pancreatic Cancer Diagnostics Market Analysis and Insights
The growing prevalence of pancreatic cancer as well as increasing need for diagnostic products for these conditions have enhanced the market demand. The advancement in technology for easy supply of products and fast manufacturing facilities are also attributing in the growth of the market. The major market players are highly focusing on product launches and product approvals during this crucial period. In addition, the government and regulatory bodies are supporting market players by product approval due to surging emergence.
The North America pancreatic cancer diagnostics market is expected to grow in the forecast year due to the rise in market players and the availability of advanced services. Along with this, manufacturers are engaged in R&D activity for launching novel services in the market. The increasing research in the field of leukemia diagnosis and development is expected to further boost the market growth. However, difficulties in leukemia screening techniques is expected to hamper the growth of the North America pancreatic cancer diagnostics market in the forecast period. Increasing healthcare expenditure on cancer diagnosis and treatment is expected to give opportunities to the market to enhance the treatment. The improvement in awareness about regular healthcare checkups, upcoming diagnostic centers and advancements in diagnostic methods for pancreatic cancer and technological developments is expected to boost the market’s growth. However, the high cost of testing and strict regulations and standards for the approval and commercialization of cancer diagnostic products and instruments is expected to challenge market growth.
La croissance de la population gériatrique, les initiatives stratégiques des acteurs du marché et du gouvernement, ainsi que l'augmentation des dépenses de santé offrent au marché des opportunités d'améliorer le traitement. Cependant, le manque de professionnels qualifiés et les cadres réglementaires stricts constituent des défis majeurs pour la croissance du marché. Cependant, le coût élevé des appareils et des traitements devrait freiner la croissance du marché nord-américain du diagnostic du cancer du pancréas.
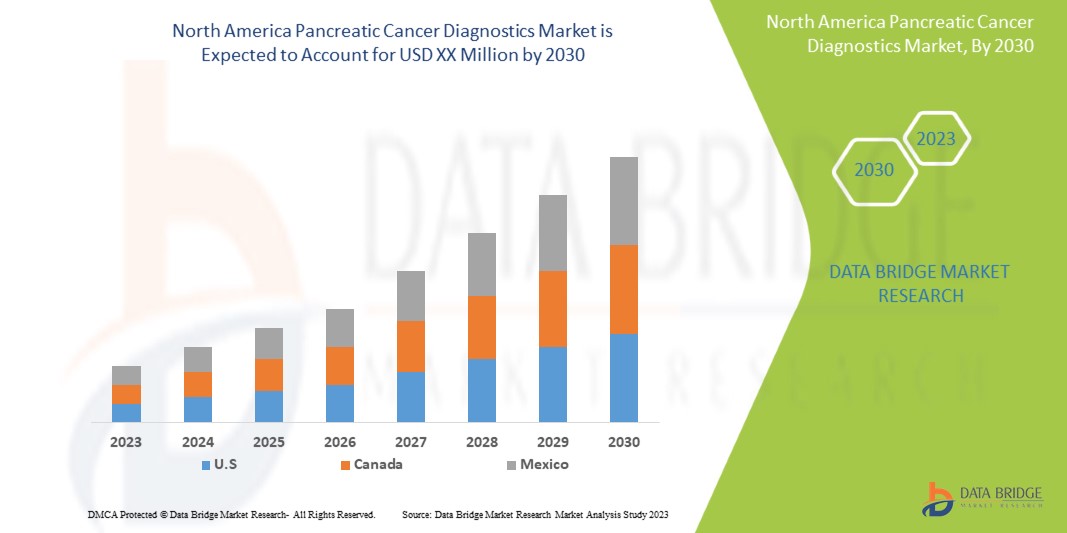
Le marché nord-américain du diagnostic du cancer du pancréas est favorable et vise à réduire la maladie, améliorant ainsi la récupération et les performances des individus. Data Bridge Market Research analyse que le marché nord-américain du diagnostic du cancer du pancréas connaîtra une croissance de 7,7 % au cours de la période de prévision de 2023 à 2030.
|
Rapport métrique |
Détails |
|
Période de prévision |
2023 à 2030 |
|
Année de base |
2022 |
|
Années historiques |
2021 (personnalisable pour 2020-2016) |
|
Unités quantitatives |
Chiffre d'affaires en millions USD, prix en USD |
|
Segments couverts |
Par type de test (test d'imagerie, biopsie, test sanguin, test génomique et autres), stade du cancer (stade 0, stade I, stade II, stade III et stade IV), type de tumeur (tumeurs exocrines et tumeurs neuroendocrines ), produit (produits basés sur des instruments, produits basés sur une plateforme, kits et réactifs et autres consommables), technologie (hybridation in situ fluorescente, séquençage de nouvelle génération, fluoroimmuno-essai, hybridation génomique comparative, immunohistochimique et autres), application (dépistage, diagnostic et prédictif, pronostic et recherche), utilisateur final (hôpitaux, centres de diagnostic, centres de recherche sur le cancer, instituts universitaires, centres de chirurgie ambulatoire et autres), canal de distribution (appel d'offres direct, vente au détail et autres). |
|
Pays couvert |
États-Unis, Canada, Mexique. |
|
Acteurs du marché couverts |
Siemens Healthcare Private Limited, Koninklijke Philips NV, FUJIFILM Corporation, Grail, Laboratory Corporation of America Holdings, DiaSource, Abbott, Agilent Technologies, Inc., Lee Biosolutions, Inc, MP BIOMEDICALS, Setia Scientific Solution, Boditech Med Inc., AccuBioTech Co., Ltd., Thermo Fisher Scientific, Creative Biolabs, Myriad Genetics, Inc., BD, CANON MEDICAL SYSTEMS CORPORATION, QIAGEN, Meridian Life Science, Inc., CTK Biotech, Inc., entre autres |
Définition du marché
Le cancer du pancréas est mortel et le processus de diagnostic du cancer du pancréas présente également des problèmes de sécurité ; il n’est pas rentable. L’un des troubles médicaux les plus coûteux à traiter en Amérique du Nord est le cancer. Les patients atteints de cancer peuvent être hospitalisés et recevoir une variété de thérapies pour la tumeur, comme la chirurgie, la radiothérapie et la thérapie systémique. Les primes d’assurance maladie pour les patients atteints de cancer sont désormais plus chères que par le passé. De plus, leurs frais de co-paiement, de franchise et de coassurance augmentent. Le diagnostic du cancer du pancréas comprend des échographies, des biopsies et des analyses sanguines. Le cancer du pancréas est l’une des principales causes de décès dans le monde et la prévalence de cette maladie a augmenté à un rythme alarmant.
Dynamique du marché du diagnostic du cancer du pancréas en Amérique du Nord
Cette section traite de la compréhension des moteurs, des opportunités, des contraintes et des défis du marché. Tous ces éléments sont abordés en détail ci-dessous :
Conducteurs
- Augmentation de la prévalence du cancer du pancréas
Ce type de cancer peut toucher des personnes de tous âges. Il peut être difficile à diagnostiquer car, malgré sa large gamme de signes et de symptômes, ils ne sont pas spécifiques et peuvent être liés à d'autres pathologies plus répandues. Le cancer du pancréas est le huitième cancer le plus fréquent chez les femmes et le dixième cancer le plus fréquent chez les hommes. Les taux d'incidence du cancer du pancréas ont augmenté d'environ 1 % chaque année. Il survient moins fréquemment. Il est légèrement plus fréquent chez les femmes que chez les hommes, mais le risque moyen de développer un cancer du pancréas au cours de la vie chez les deux sexes est d'environ 0,5 % en moyenne. Ces pathologies comprennent : des douleurs abdominales, une perte d'appétit ou une perte de poids involontaire, un jaunissement de la peau et du blanc des yeux (jaunisse), des selles claires, des urines foncées et des démangeaisons cutanées. Il s'agit du huitième type de cancer le plus fréquemment diagnostiqué chez les adultes et les enfants, mais la plupart des cas surviennent chez les adultes. Bien qu'il puisse être diagnostiqué à tout âge, il est rare avant 45 ans. L'âge moyen du diagnostic est de 68 ans.
En raison de divers facteurs de risque, l'incidence du cancer du pancréas est en hausse en Amérique du Nord, devenant un problème socio-économique majeur. Cela devrait agir comme un moteur sur le marché nord-américain du diagnostic du cancer du pancréas.
- De nouvelles avancées technologiques dans le diagnostic du pancréas
Le cancer du pancréas est rarement détecté à un stade précoce, lorsqu'il est le plus facilement curable. En effet, il n'entraîne souvent aucun symptôme avant de s'être propagé à d'autres organes. Les spécialistes doivent diagnostiquer manuellement les cellules cancéreuses et non cancéreuses en examinant les images cellulaires au microscope et en les annotant. Cependant, cet examen microscopique manuel prend du temps et peut donner un diagnostic erroné. Le risque de prescrire des médicaments inappropriés a alors été réduit grâce à un logiciel informatisé. La création d'un système de classification automatique et fiable est devenue essentielle pour mettre fin aux effets dévastateurs de la maladie du pancréas. Les techniques de segmentation multiples constituent la base des algorithmes de classification du cancer du pancréas existants.
Opportunité
- Augmentation des dépenses de santé pour le diagnostic et le traitement du cancer
Partout dans le monde, les activités de recherche et développement augmentent en raison des dépenses de santé publique et des performances économiques. Alors que le secteur de la santé se classe au deuxième rang parmi tous les secteurs en ce qui concerne le montant dépensé pour les soins de santé, l'augmentation des dépenses de santé peut entraîner une meilleure offre d'opportunités de recherche et développement. On s'attend à ce qu'elle augmente la demande de diagnostics du cancer du pancréas. L'augmentation des dépenses de santé pour le traitement du cancer du pancréas aide également le patient à obtenir des diagnostics et des traitements avancés sans tracas pour une guérison rapide. Les dépenses de santé sont constituées de la combinaison des paiements directs (les personnes paient pour leurs soins), des dépenses gouvernementales et des sources. Cela comprend également l'assurance maladie et les activités des organisations non gouvernementales. Cette augmentation des dépenses de santé pour le traitement du cancer est une opportunité pour la demande du marché.
Retenue/Défi
- Diagnostic tardif et mauvais pronostic du cancer du pancréas
Le diagnostic tardif de la maladie est dû à l’augmentation du nombre de tumeurs cancéreuses du pancréas qui ne répondent pas aussi bien aux thérapies anticancéreuses couramment utilisées que d’autres types de cancer moins mortels. Il existe néanmoins des options de traitement, notamment la chirurgie, la chimiothérapie et la radiothérapie. Il existe différents types de cancer du pancréas. La plupart des cancers du pancréas sont de type exocrine. Cela signifie qu’ils commencent dans les cellules qui produisent les sucs digestifs pancréatiques. Environ 30 % des patients sont fumeurs et 5 % ont des antécédents de pancréatite, une inflammation du pancréas qui peut être provoquée par des calculs ou une forte consommation d’alcool.
Impact de la pandémie de COVID-19 sur le marché nord-américain du diagnostic du cancer du pancréas
La COVID-19 a eu un impact négatif sur la croissance du marché, car les patients souffrant d'un cancer du pancréas ont reporté leur intervention chirurgicale en raison de l'augmentation rapide des cas de COVID-19 dans toutes les régions du monde. De plus, les personnes atteintes d'un cancer du pancréas risquaient de tomber gravement malades. La peur de l'infection par le coronavirus a affecté la croissance du marché du diagnostic du cancer du pancréas en pleine pandémie.
Développements récents
- En décembre 2022, FUJIFILM Holdings America Corporation a annoncé que la société avait conclu un accord d'achat d'actifs avec Inspirata, Inc. pour acquérir une activité de pathologie numérique afin d'élargir son offre d'imagerie d'entreprise robuste. Cela permet l'intégration d'images et de données de pathologie dans le système de dossiers médicaux électroniques d'un établissement de santé afin de rationaliser la prestation de soins pour les patients atteints de cancer.
- En août 2020, Siemens Healthcare GmbH a annoncé avoir conclu un accord avec Varian Medical Systems, Inc. Avec cette acquisition, Siemens Healthcare a contribué au développement de solutions avancées pour traiter le cancer et renforcer sa position dans le secteur de la santé.
Portée du marché nord-américain du diagnostic du cancer du pancréas
Le marché nord-américain du diagnostic du cancer du pancréas est divisé en huit segments notables en fonction du type de test, des stades du cancer, du type de tumeur, du produit, de l'application, de la technologie, de l'utilisateur final et du canal de distribution. La croissance entre les segments vous aide à analyser les niches de croissance et les stratégies pour aborder le marché et déterminer vos principaux domaines d'application et la différence entre vos marchés cibles.
Type de test
- Test d'imagerie
- Biopsie
- Analyse de sang
- Test génomique
- Autres
Sur la base du type de test, le marché nord-américain du diagnostic du cancer du pancréas est segmenté en test d'imagerie, biopsie, test sanguin, test génomique et autres.
Stade du cancer
- Étape 0
- Étape I
- Stade II
- Stade III
- Stade IV
Sur la base du stade du cancer, le marché nord-américain du diagnostic du cancer du pancréas est segmenté en stade 0, stade I, stade II, stade III et stade IV.
Type de tumeur
- Tumeurs exocrines
- Tumeurs neuroendocrines
Sur la base du type de tumeur, le marché nord-américain du diagnostic du cancer du pancréas est segmenté en tumeurs exocrines et tumeurs neuroendocrines.
Produit
- Produits basés sur des instruments
- Produits basés sur une plateforme
- Kits et réactifs
- Autres consommables
Sur la base du produit, le marché nord-américain du diagnostic du cancer du pancréas est segmenté en produits basés sur des instruments, produits basés sur des plates-formes, kits et réactifs et autres consommables.
Application
- Dépistage
- Diagnostic et prédictif
- Pronostic
- Recherche
Sur la base des applications, le marché nord-américain du diagnostic du cancer du pancréas est segmenté en dépistage, diagnostic et prédictif, pronostic et recherche.
Technologie
- Hybridation in situ fluorescente
- Séquençage de nouvelle génération
- Dosage fluoro-immunologique
- Hybridation génomique comparative
- Immunohistochimique
- Autres
Sur la base de la technologie, le marché nord-américain du diagnostic du cancer du pancréas est segmenté en hybridation fluorescente in situ, séquençage de nouvelle génération, fluoroimmuno-essai, hybridation génomique comparative, immunohistochimie et autres.
Utilisateur final
- Hôpitaux
- Centres de diagnostic
- Centres de recherche sur le cancer
- Instituts universitaires
- Centres de chirurgie ambulatoire
- Autres
Sur la base de l'utilisateur final, le marché nord-américain du diagnostic du cancer du pancréas est segmenté en hôpitaux, centres de diagnostic, centres de recherche sur le cancer, instituts universitaires, centres de chirurgie ambulatoire et autres.
Canal de distribution
- Appel d'offres direct
- Ventes au détail
- Autres
Sur la base du canal de distribution, le marché nord-américain du diagnostic du cancer du pancréas est segmenté en appels d'offres directs, ventes au détail et autres.
Analyse/perspectives du marché nord-américain du diagnostic du cancer du pancréas
Le marché nord-américain du diagnostic du cancer du pancréas est analysé et des informations et tendances sur la taille du marché sont fournies par pays, type de test, stades du cancer, type de tumeur, produit, application, technologie, utilisateur final et canal de distribution comme référencé ci-dessus.
- In 2023, U.S. pancreatic cancer diagnostics market is expected to grow due to rise in prevalence and incidence of pancreatic cancer and increase in awareness about the pancreatic cancer diagnostics. These are the key contributing factors which is expected to boost the growth of the market in the country.
The country section of the report also provides individual market-impacting factors and changes in market regulation that impact the current and future trends of the market. Data points such as downstream and upstream value chain analysis, technical trends, porter's five forces analysis, and case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of brands and their challenges faced due to large or scarce competition from local and domestic brands, and the impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and North America Pancreatic Cancer Diagnostics Market Share Analysis
North America pancreatic cancer diagnostics market competitive landscape provides details by the competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance. The above data points provided are only related to the company’s focus on the North America pancreatic cancer diagnostics market.
Some of the major players operating in the market are Siemens Healthcare Private Limited, Koninklijke Philips N.V., FUJIFILM Corporation, Grail, Laboratory Corporation of America Holdings, DiaSource, Abbott, Agilent Technologies, Inc., Lee Biosolutions, Inc, MP BIOMEDICALS, Setia Scientific Solution, Boditech Med Inc., AccuBioTech Co., Ltd., Thermo Fisher Scientific, Creative Biolabs, Myriad Genetics, Inc., BD, CANON MEDICAL SYSTEMS CORPORATION, QIAGEN, Meridian Life Science, Inc., CTK Biotech, Inc., among others.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 PRODUCT LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES MODEL
5 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, INDUSTRY INSIGHTS
6 EPIDEMIOLOGY
7 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, REGULATIONS
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 GROW IN PREVALENCE OF PANCREATIC CANCER
8.1.2 NOVEL TECHNOLOGICAL ADVANCEMENTS IN PANCREATIC DIAGNOSTICS
8.1.3 RISING PREFERENCE FOR PREVENTIVE HEALTH CHECK-UPS
8.1.4 INCREASE IN AWARENESS REGARDING PANCREATIC CANCER
8.2 RESTRAINTS
8.2.1 STRICT REGULATIONS AND STANDARDS FOR THE APPROVAL AND COMMERCIALIZATION OF PANCREATIC CANCER DIAGNOSTIC PRODUCTS
8.2.2 LATE DIAGNOSIS AND POOR PROGNOSIS OF PANCREATIC CANCER
8.3 OPPORTUNITIES
8.3.1 INCREASE IN DIAGNOSTIC PRODUCTS FOR PANCREATIC CANCER
8.3.2 RISE IN HEALTHCARE EXPENDITURE FOR CANCER DIAGNOSIS AND TREATMENT
8.3.3 GOVERNMENT INITIATIVES TOWARD PANCREATIC CANCER DIAGNOSTICS
8.4 CHALLENGES
8.4.1 INCREASED COST, SAFETY, AND CONVENIENCE ISSUES
8.4.2 LACK OF SKILLED AND CERTIFIED PROFESSIONALS
9 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE
9.1 OVERVIEW
9.2 IMAGING TEST
9.2.1 COMPUTED TOMOGRAPHY (CT) SCAN
9.2.2 MAGNETIC RESONANCE IMAGING (MRI)
9.2.2.1 MR CHOLANGIOPANCREATOGRAPHY
9.2.2.2 MR ANGIOGRAPHY (MRA)
9.2.3 ULTRASOUND
9.2.3.1 ABDOMINAL ULTRASOUND
9.2.3.2 ENDOSCOPIC ULTRASOUND (EUS)
9.2.4 CHOLANGIOPANCREATOGRAPHY
9.2.4.1 MAGNETIC RESONANCE CHOLANGIOPANCREATOGRAPHY (MRCP)
9.2.4.2 ENDOSCOPIC RETROGRADE CHOLANGIOPANCREATOGRAPHY (ERCP)
9.2.4.3 PRECUTANEOUS TRANSHEPTIC CHOLANGIOPANCREATOGRAPHY (PTC)
9.2.5 POSITRON EMISSION TOMOHRAPHY (PET)
9.2.6 OTHERS
9.3 BIOPSY
9.3.1 CT-GUIDED NEEDLE BIOPSY
9.3.2 FINE NEEDLE ASPIRATION (FNA)
9.3.3 CORE NEEDLE BIOPSY
9.3.4 OTHERS
9.4 BLOOD TEST
9.4.1 LIVER FUNCTION TEST
9.4.2 TUMOR MARKER
9.4.2.1 CA 19-9 BIOMARKER TEST
9.4.2.2 CARCINOEMBROYNIC ANTIGEN (CEA) TEST
9.4.2.3 CA 50 MARKER TEST
9.4.2.4 OTHERS
9.4.3 OTHERS
9.5 GENOMIC TEST
9.6 OTHERS
10 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGES
10.1 OVERVIEW
10.2 STAGE IV
10.3 STAGE III
10.4 STAGE II
10.4.1 STAGE IIA
10.4.2 STAGE IIB
10.5 STAGE I
10.5.1 STAGE IA
10.5.2 STAGE IB
10.6 STAGE 0
11 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE
11.1 OVERVIEW
11.2 EXOCRINE TUMORS
11.2.1 INSTRUMENT-BASED PRODUCTS
11.2.2 PLATFORM-BASED PRODUCTS
11.2.3 KITS AND REAGENTS
11.2.4 OTHER CONSUMABLES
11.3 NEUROENDOCRINE TUMORS
11.3.1 INSTRUMENT-BASED PRODUCTS
11.3.2 PLATFORM-BASED PRODUCTS
11.3.3 KITS AND REAGENTS
11.3.4 OTHER CONSUMABLES
12 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT
12.1 OVERVIEW
12.2 INSTRUMENT-BASED PRODUCTS
12.2.1 IMAGING
12.2.2 BIOPSY
12.3 PLATFORM-BASED PRODUCTS
12.3.1 NEXT-GENERATION SEQUENCING
12.3.2 MICROARRAYS
12.3.3 PCR
12.3.4 OTHERS
12.4 KITS AND REAGENTS
12.4.1 CA19-9 PANCREATIC CANCER TEST KITS
12.4.1.1 ELISA TEST KITS
12.4.1.2 CASETTE TEST KITS
12.4.1.3 OTHERS
12.4.2 CEA PANCREATIC CANCER TEST KITS
12.4.2.1 ELISA TEST KITS
12.4.2.2 CASETTE TEST KITS
12.4.2.3 OTHERS
12.5 OTHER CONSUMABLES
13 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY
13.1 OVERVIEW
13.2 FLUORESCENT IN SITU HYBRIDIZATION
13.3 NEXT GENERATION SEQUENCING
13.4 FLUORIMMUNOASSAY
13.5 COMPARATIVE GENOMIC HYBRIDIZATION
13.6 IMMUNOHISTOCHEMICAL
13.7 OTHERS
14 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION
14.1 OVERVIEW
14.2 SCREENING
14.2.1 INSTRUMENT-BASED PRODUCTS
14.2.2 PLATFORM-BASED PRODUCTS
14.2.3 KITS AND REAGENTS
14.2.4 OTHER CONSUMABLES
14.3 DIAGNOSTIC AND PREDICTIVE
14.3.1 INSTRUMENT-BASED PRODUCTS
14.3.2 PLATFORM-BASED PRODUCTS
14.3.3 KITS AND REAGENTS
14.3.4 OTHER CONSUMABLES
14.4 PROGNOSTIC
14.4.1 INSTRUMENT-BASED PRODUCTS
14.4.2 PLATFORM-BASED PRODUCTS
14.4.3 KITS AND REAGENTS
14.4.4 OTHER CONSUMABLES
14.5 RESEARCH
14.5.1 INSTRUMENT-BASED PRODUCTS
14.5.2 PLATFORM-BASED PRODUCTS
14.5.3 KITS AND REAGENTS
14.5.4 OTHER CONSUMABLES
15 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER
15.1 OVERVIEW
15.2 HOSPITALS
15.3 DIAGNOSTIC CENTERS
15.4 CANCER RESEARCH CENTERS
15.5 ACADEMIC INSTITUTES
15.6 AMBULATORY SURGICAL CENTERS
15.7 OTHERS
16 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
16.1 OVERVIEW
16.2 DIRECT TENDER
16.3 RETAIL SALES
16.4 OTHERS
17 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION
17.1 NORTH AMERICA
17.1.1 U.S.
17.1.2 CANADA
17.1.3 MEXICO
18 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: COMPANY LANDSCAPE
18.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
19 SWOT ANALYSIS
20 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET
20.1 CANON MEDICAL SYSTEMS CORPORATION
20.1.1 COMPANY SNAPSHOT
20.1.2 REVENUE ANALYSIS
20.1.3 COMPANY SHARE ANALYSIS
20.1.4 PRODUCT PORTFOLIO
20.1.5 RECENT DEVELOPMENT
20.2 KONINKLIJKE PHILIPS N.V.
20.2.1 COMPANY SNAPSHOT
20.2.2 REVENUE ANALYSIS
20.2.3 COMPANY SHARE ANALYSIS
20.2.4 PRODUCT PORTFOLIO
20.2.5 RECENT DEVELOPMENTS
20.3 SIEMENS HEALTHCARE GMBH
20.3.1 COMPANY SNAPSHOT
20.3.2 REVENUE ANALYSIS
20.3.3 COMPANY SHARE ANALYSIS
20.3.4 PRODUCT PORTFOLIO
20.3.5 RECENT DEVELOPMENT
20.4 GRAIL
20.4.1 COMPANY PROFILE
20.4.2 COMPANY SHARE ANALYSIS
20.4.3 PRODUCT PORTFOLIO
20.4.4 RECENT DEVELOPMENT
20.5 MYRIAD GENETICS, INC.
20.5.1 COMPANY SNAPSHOT
20.5.2 REVENUE ANALYSIS
20.5.3 COMPANY SHARE ANALYSIS
20.5.4 PRODUCT PORTFOLIO
20.5.5 RECENT DEVELOPMENT
20.6 BD
20.6.1 COMPANY SNAPSHOT
20.6.2 REVENUE ANALYSIS
20.6.3 PRODUCT PORTFOLIO
20.6.4 RECENT DEVELOPMENT
20.7 BODITECH MED INC.
20.7.1 COMPANY PROFILE
20.7.2 PRODUCT PORTFOLIO
20.7.3 RECENT DEVELOPMENT
20.8 ABBOTT (2022)
20.8.1 COMPANY SNAPSHOT
20.8.2 REVENUE ANALYSIS
20.8.3 PRODUCT PORTFOLIO
20.8.4 RECENT DEVELOPMENT
20.9 FUJIFILM HOLDINGS AMERICA CORPORATION
20.9.1 COMPANY SNAPSHOT
20.9.2 REVENUE ANALYSIS
20.9.3 PRODUCT PORTFOLIO
20.9.4 RECENT DEVELOPMENT
20.1 ACCUBIOTECH CO., LTD.
20.10.1 COMPANY PROFILE
20.10.2 PRODUCT PORTFOLIO
20.10.3 RECENT DEVELOPMENTS
20.11 AGILENT TECHNOLOGIES, INC.
20.11.1 COMPANY PROFILE
20.11.2 REVENUE ANALYSIS
20.11.3 PRODUCT PORTFOLIO
20.11.4 RECENT DEVELOPMENT
20.12 CREATIVE BIOLABS.
20.12.1 COMPANY PROFILE
20.12.2 PRODUCT PORTFOLIO
20.12.3 RECENT DEVELOPMENT
20.13 CTK BIOTECH, INC.
20.13.1 COMPANY PROFILE
20.13.2 PRODUCT PORTFOLIO
20.13.3 RECENT DEVELOPMENT
20.14 DIASOURCE
20.14.1 COMPANY SNAPSHOT
20.14.2 PRODUCT PORTFOLIO
20.14.3 RECENT DEVELOPMENT
20.15 LABORATORY CORPORATION OF AMERICA HOLDINGS
20.15.1 COMPANY SNAPSHOT
20.15.2 REVENUE ANALYSIS
20.15.3 PRODUCT PORTFOLIO
20.15.4 RECENT DEVELOPMENTS
20.16 LEE BIOSCIENCE
20.16.1 COMPANY SNAPSHOT
20.16.2 PRODUCT PORTFOLIO
20.16.3 RECENT DEVELOPMENT
20.17 MERIDIAN BIOSCIENCE INC.
20.17.1 COMPANY PROFILE
20.17.2 PRODUCT PORTFOLIO
20.17.3 RECENT DEVELOPMENT
20.18 MP BIOMEDICALS.
20.18.1 COMPANY PROFILE
20.18.2 PRODUCT PORTFOLIO
20.18.3 RECENT DEVELOPMENTS
20.19 QIAGEN
20.19.1 COMPANY SNAPSHOT
20.19.2 REVENUE ANALYSIS
20.19.3 PRODUCT PORTFOLIO
20.19.4 RECENT DEVELOPMENT
20.2 SETIA SCIENTIFIC SOLUTION
20.20.1 COMPANY PROFILE
20.20.2 PRODUCT PORTFOLIO
20.20.3 RECENT DEVELOPMENTS
20.21 THERMO FISHER SCIENTIFIC INC.
20.21.1 COMPANY SNAPSHOT
20.21.2 REVENUE ANALYSIS
20.21.3 PRODUCT PORTFOLIO
20.21.4 RECENT DEVELOPMENT
21 QUESTIONNAIRE
22 RELATED REPORTS
Liste des tableaux
TABLE 1 APPROVED DIAGNOSTICS OF PANCREATIC CANCER
TABLE 2 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 3 NORTH AMERICA IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA MAGNETIC RESONANCE IMAGING (MRI) IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA ULTRASOUND IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA CHOLANGIOPANCREATOGRAPHY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA TUMOR MARKER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA GENOMIC TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA OTHERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGES, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA STAGE IV IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA STAGE III IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA STAGE 0 IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 25 NORTH AMERICA EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 26 NORTH AMERICA NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 27 NORTH AMERICA NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 28 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 29 NORTH AMERICA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 NORTH AMERICA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 31 NORTH AMERICA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 NORTH AMERICA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 33 NORTH AMERICA KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 34 NORTH AMERICA KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 35 NORTH AMERICA CA19-9 PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 36 NORTH AMERICA CEA PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 37 NORTH AMERICA OTHER CONSUMABLES IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 38 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 39 NORTH AMERICA FLUORESCENT IN SITU HYBRIDIZATION IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 40 NORTH AMERICA NEXT GENERATION SEQUENCING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 41 NORTH AMERICA FLUORIMMUNOASSAY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 42 NORTH AMERICA COMPARATIVE GENOMIC HYBRIDIZATION IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 43 NORTH AMERICA IMMUNOHISTOCHEMICAL IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 44 NORTH AMERICA OTHERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 45 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 46 NORTH AMERICA SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 47 NORTH AMERICA SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 48 NORTH AMERICA DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 49 NORTH AMERICA DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 50 NORTH AMERICA PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 51 NORTH AMERICA PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 52 NORTH AMERICA RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 53 NORTH AMERICA RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 54 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 55 NORTH AMERICA HOSPITALS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 56 NORTH AMERICA DIAGNOSTIC CENTERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 57 NORTH AMERICA CANCER RESEARCH CENTERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 58 NORTH AMERICA ACADEMIC INSTITUTES IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 59 NORTH AMERICA AMBULATORY SURGICAL CENTERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 60 NORTH AMERICA OTHERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 61 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 62 NORTH AMERICA DIRECT TENDER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 63 NORTH AMERICA RETAIL SALES IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 64 NORTH AMERICA OTHERS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 65 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 66 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 67 NORTH AMERICA IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 68 NORTH AMERICA MAGNETIC RESONANCE IMAGING (MRI) IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 69 NORTH AMERICA ULTRASOUND IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 70 NORTH AMERICA CHOLANGIOPANCREATOGRAPHY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 71 NORTH AMERICA BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 72 NORTH AMERICA TUMOR MARKER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 73 NORTH AMERICA BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 74 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 75 NORTH AMERICA STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 76 NORTH AMERICA STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 77 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 78 NORTH AMERICA EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 79 NORTH AMERICA NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 80 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 81 NORTH AMERICA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 82 NORTH AMERICA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 83 NORTH AMERICA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 84 NORTH AMERICA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 85 NORTH AMERICA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 86 NORTH AMERICA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 87 NORTH AMERICA KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 88 NORTH AMERICA CA19-9 PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 89 NORTH AMERICA CEA PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 90 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 91 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 92 NORTH AMERICA SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 93 NORTH AMERICA DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 94 NORTH AMERICA PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 95 NORTH AMERICA RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 96 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 97 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 98 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 99 U.S. IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 100 U.S. MAGNETIC RESONANCE IMAGING (MRI) IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 101 U.S. ULTRASOUND IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 102 U.S. CHOLANGIOPANCREATOGRAPHY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 103 U.S. BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 104 U.S. TUMOR MARKER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 105 U.S. BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 106 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 107 U.S. STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 108 U.S. STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 109 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 110 U.S. EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 111 U.S. NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 112 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 113 U.S. INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 114 U.S. INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 115 U.S. INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 116 U.S. PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 117 U.S. PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 118 U.S. PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 119 U.S. KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 120 U.S. CA19-9 PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 121 U.S. CEA PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 122 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 123 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 124 U.S. SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 125 U.S. DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 126 U.S. PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 127 U.S. RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 128 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 129 U.S. PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 130 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 131 CANADA IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 132 CANADA MAGNETIC RESONANCE IMAGING (MRI) IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 133 CANADA ULTRASOUND IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 134 CANADA CHOLANGIOPANCREATOGRAPHY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 135 CANADA BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 136 CANADA TUMOR MARKER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 137 CANADA BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 138 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 139 CANADA STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 140 CANADA STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 141 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 142 CANADA EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 143 CANADA NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 144 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 145 CANADA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 146 CANADA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 147 CANADA INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 148 CANADA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 149 CANADA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 150 CANADA PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 151 CANADA KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 152 CANADA CA19-9 PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 153 CANADA CEA PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 154 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 155 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 156 CANADA SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 157 CANADA DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 158 CANADA PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 159 CANADA RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 160 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 161 CANADA PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 162 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 163 MEXICO IMAGING TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 164 MEXICO MAGNETIC RESONANCE IMAGING (MRI) IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 165 MEXICO ULTRASOUND IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 166 MEXICO CHOLANGIOPANCREATOGRAPHY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 167 MEXICO BLOOD TEST IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 168 MEXICO TUMOR MARKER IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 169 MEXICO BIOPSY IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 170 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 171 MEXICO STAGE I IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 172 MEXICO STAGE II IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 173 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 174 MEXICO EXOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 175 MEXICO NEUROENDOCRINE TUMORS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 176 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 177 MEXICO INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 178 MEXICO INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 179 MEXICO INSTRUMENT-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 180 MEXICO PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 181 MEXICO PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 182 MEXICO PLATFORM-BASED PRODUCTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 183 MEXICO KITS AND REAGENTS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 184 MEXICO CA19-9 PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 185 MEXICO CEA PANCREATIC CANCER TEST KITS IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 186 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 187 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 188 MEXICO SCREENING IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 189 MEXICO DIAGNOSTIC AND PREDICTIVE IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 190 MEXICO PROGNOSTIC IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 191 MEXICO RESEARCH IN PANCREATIC CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 192 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 193 MEXICO PANCREATIC CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
Liste des figures
FIGURE 1 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: DBMR MARKET POSITION GRID
FIGURE 9 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 11 GROWING AWARENESS OF PANCREATIC CANCER AND INCREASING HEALTHCARE EXPENDITURE IS EXPECTED TO DRIVE THE GROWTH OF THE NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET FROM 2023 TO 2030
FIGURE 12 IMAGING TEST SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET
FIGURE 14 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TEST TYPE, 2022
FIGURE 15 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TEST TYPE, 2023-2030 (USD MILLION)
FIGURE 16 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TEST TYPE, CAGR (2023-2030)
FIGURE 17 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 18 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY CANCER STAGES, 2022
FIGURE 19 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY CANCER STAGES, 2023-2030 (USD MILLION)
FIGURE 20 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY CANCER STAGES, CAGR (2023-2030)
FIGURE 21 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY CANCER STAGES, LIFELINE CURVE
FIGURE 22 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TUMOR TYPE, 2022
FIGURE 23 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TUMOR TYPE, 2023-2030 (USD MILLION)
FIGURE 24 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TUMOR TYPE, CAGR (2023-2030)
FIGURE 25 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TUMOR TYPE, LIFELINE CURVE
FIGURE 26 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY PRODUCT, 2022
FIGURE 27 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY PRODUCT, 2023-2030 (USD MILLION)
FIGURE 28 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY PRODUCT, CAGR (2023-2030)
FIGURE 29 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 30 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TECHNOLOGY, 2022
FIGURE 31 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TECHNOLOGY, 2023-2030 (USD MILLION)
FIGURE 32 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TECHNOLOGY, CAGR (2023-2030)
FIGURE 33 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY TECHNOLOGY, LIFELINE CURVE
FIGURE 34 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY APPLICATION, 2022
FIGURE 35 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY APPLICATION, 2023-2030 (USD MILLION)
FIGURE 36 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY APPLICATION, CAGR (2023-2030)
FIGURE 37 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 38 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY END USER, 2022
FIGURE 39 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 40 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 41 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY END USER, LIFELINE CURVE
FIGURE 42 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 43 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 44 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 45 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 46 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 47 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 48 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 49 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 50 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: CATEGORY (2023-2030)
FIGURE 51 NORTH AMERICA PANCREATIC CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.