北米の甲状腺がん診断市場、製品タイプ別(機器、消耗品、付属品)、検査タイプ別(画像検査、生検、血液検査、その他)、がんの種類別(乳頭がん、濾胞がん、その他)、ステージ別(ステージ I、ステージ II、ステージ III、ステージ IV)、年齢層別(21 歳未満、21~29 歳、30~65 歳、65 歳以上)、エンドユーザー別(病院、関連研究所、独立診断研究所、診断画像センター、がん研究機関、その他)、流通チャネル別(直接入札、小売販売) - 2030 年までの業界動向と予測。
北米の甲状腺がん診断市場の分析と洞察
甲状腺がんに対する意識の高まりにより、市場の需要が高まっています。より良い医療サービスを求める医療費の増加も、市場の成長に貢献しています。主要な市場プレーヤーは、この重要な時期にさまざまなサービスの立ち上げと承認に重点を置いています。さらに、診断プロセスと技術の改善の増加も、甲状腺がんの診断検査の需要の増加に貢献しています。
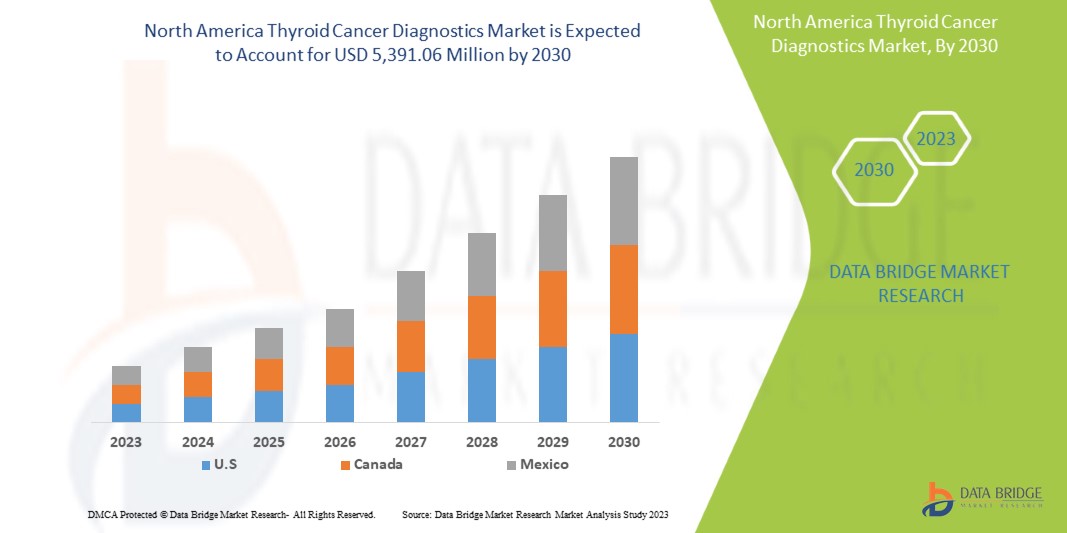
北米の甲状腺がん診断市場は、2023年から2030年の予測期間に成長すると予想されています。データブリッジマーケットリサーチは、市場は2023年から2030年の予測期間に6.4%のCAGRで成長し、2030年までに53億9,106万米ドルに達すると分析しています。
|
レポートメトリック |
詳細 |
|
予測期間 |
2023年から2030年 |
|
基準年 |
2022 |
|
歴史的な年 |
2021 (2020~2015年にカスタマイズ可能) |
|
定量単位 |
収益(百万米ドル) |
|
対象セグメント |
製品タイプ別(機器、消耗品、付属品)、検査タイプ別(画像検査、生検、血液検査、その他)、がんの種類別(乳頭がん、濾胞がん、その他)、ステージ別(ステージ I、ステージ II、ステージ III、ステージ IV)、年齢層別(21 歳未満、21~29 歳、30~65 歳、65 歳以上)、エンドユーザー別(病院、関連ラボ、独立診断ラボ、診断画像センター、がん研究機関、その他)、流通チャネル別(直接入札、小売販売) |
|
対象国 |
米国、カナダ、メキシコ |
|
対象となる市場プレーヤー |
キヤノン株式会社、富士フイルムホールディングス株式会社、F. ホフマン・ラ・ロシュ株式会社、クエスト・ダイアグノスティクス株式会社、イルミナ、フィリップス社、サーモフィッシャーサイエンティフィック株式会社、シーメンスヘルスケア株式会社、アボット、ゼネラルエレクトリックカンパニー、BD、キアゲン、ディアソリンSPA、メルクKGaA、ホロジック、ミリアドジェネティクス株式会社、ビオメリュー、FONAR株式会社、タイムメディカルホールディングス、プレックスバイオ、ミンファウンドメディカルシステムズ株式会社、メドニカ株式会社、北京O&Dバイオテック株式会社、シュテルンメッド株式会社など |
市場の定義
甲状腺がんは、甲状腺で発生するがんの一種です。がんは、細胞が制御不能に増殖し始めると発生します。甲状腺は、代謝、心拍数、血圧、体温の調節を助けるホルモンを生成します。甲状腺は首の前部、甲状腺軟骨 (喉仏) の下にあります。ほとんどの人では、甲状腺は見えず、触れることもできません。甲状腺は蝶のような形をしており、右葉と左葉の 2 つの葉が峡部と呼ばれる狭い部分でつながっています。
北米の甲状腺がん診断市場の動向
ドライバー
甲状腺結節と癌の発生率と罹患率の上昇
甲状腺結節は、甲状腺における甲状腺細胞の異常な増殖(塊)です。正常な甲状腺軟部組織が増殖し始め、これらの結節が形成されることがあります。結節の発生率は加齢とともに増加し、主に女性で、主にヨウ素欠乏症および放射線被曝後に発生します。これらの結節のさらなる合併症は甲状腺癌ですが、甲状腺結節が甲状腺癌に変化する可能性は低いです。2017年にNCBIで発表された「4cm以上の甲状腺結節の悪性腫瘍リスク」という記事によると、結節後の癌発生は、結節症例全体の5%未満で見られます。さらに、甲状腺結節は家族歴があり、ヨウ素摂取量が少ない人に発生します。
甲状腺がんの診断検査の増加
甲状腺超音波検査は、携帯型機器で甲状腺の音波画像を撮影し、モニター上で 2 次元画像に変換します。甲状腺の腫瘍、嚢胞、または甲状腺腫の診断に使用され、痛みやリスクのない処置です。これらの検査は構造的異常を評価するために使用され、TSH、T4、および T3 レベルを測定する血液検査は機能変数を調べるために使用されます。疑わしいケースでは、腫瘍が良性か悪性かを判断するために細針生検が使用されます。さらに、分子検査と遺伝的予後が導入されたことで、甲状腺がん診断のこの市場での診断環境が活性化しています。
甲状腺がんに対する意識の高まり
甲状腺がんに対する意識が高まるにつれて、タイムリーながん検出の需要が高まり、市場の成長につながっています。
甲状腺がんは、世界中の米国人の死亡率上昇の主な原因の 1 つであり、今後 5 年間の市場成長の原動力となります。放射線への曝露と甲状腺疾患の家族歴は、甲状腺がんの主な危険因子です。甲状腺がんと診断される女性は、男性よりも大幅に多くなっています。
拘束
診断手順の高額な費用
甲状腺がん患者数の増加と医療機器価格の高騰により、がん診断はますます高価になっています。がん診断に使用される最新の技術機器も、がん診断の高価格化と、甲状腺結節のがんの確定診断における高い精度に重要な役割を果たしています。そのため、甲状腺がんの診断手順の高コストが市場の成長を妨げています。
がんの検出に使用される診断製品や機器は進歩していますが、それに伴い、がんの診断手順も高価になり、甲状腺がん診断市場の成長を妨げています。がん診断のプロセスで使用される機器が高価になり、診断手順のコストが増加するためです。したがって、がん診断の診断コストの高さは、北米の甲状腺がん診断市場の抑制要因となっています。
画像検査による高放射線被曝による組織損傷
放射線に大量に被ばくすると、組織に重大な損傷が生じ、後にがんを発症するリスクが高まります。このリスクを念頭に置くことは重要ですが、画像検査に使用される微量の放射線量によって、がんを発症するリスクがわずかに高まる可能性があります。人が受ける放射線の量は、検査の種類、被ばくする体の部位、体の大きさ、年齢、性別などによって異なります。放射線を使用する画像検査は、生涯にわたってあらゆる放射線源からの放射線被ばくが蓄積し、がんを発症するリスクを高める可能性があるため、必要な場合にのみ行う必要があります。超音波や MRI などの他の画像検査も頻繁に使用される場合があります。
機会
がんの診断と治療にかかる医療費の増加
世界中で、経済パフォーマンスに伴う公衆衛生支出により研究開発活動が拡大していますが、医療業界は医療費支出額で全業界中第 2 位となっています。医療費の増加は、研究開発の機会の提供の改善につながる可能性があります。卵巣がん診断の需要が急増すると予想されます。
がん治療に対する医療費の増加は、患者が手間をかけずに高度な診断と治療を受け、早期回復するのにも役立ちます。医療費は、自己負担(患者が自分の治療費を支払う)、政府支出、健康保険や非政府組織(NGO)の活動などの資金源で構成されています。がん治療に対する医療費の増加により、市場成長の機会が生まれます。
課題
がん診断製品の承認と商業化のための厳格な規制枠組み
The stringent regulations for the approval and commercialization of any product in the market are proving to be one of the major challenges for manufacturers of cancer diagnostic products in the U.S. and European region. Every country has regulations and a different body for regulatory procedures.
The potential regulatory pathways are mandatory for clearance, approval, or acceptance of complex signatures by the U.S. Food and Drug Administration (FDA). The regulatory pathways include regulations applicable to In Vitro Diagnostic (IVD) devices, including companion diagnostic devices, the potential for labeling as a complementary diagnostic, and the biomarker qualification program.
Recent Developments
- In August 2022, F. Hoffmann-La Roche Ltd, announced the launch of the Digital LightCycler System, Roche's first digital polymerase chain reaction (PCR) system. This next-generation system detects disease and is designed to accurately quantify trace amounts of specific DNA and RNA targets not typically detectable by conventional PCR methods. This has helped the company to increase its North America presence in the market
- In May 2022, Thermo Fisher Scientific Inc., the world leader in serving science, introduced the Thermo Scientific Glacios 2 Cryo-Transmission Electron Microscope (Cryo-TEM), a powerful microscope with new automation and high-resolution imaging capabilities designed to help cryo-electron microscopy (cryo-EM) researchers of varying experience levels accelerate structure-based drug discovery. This advanced, fast, and cost-efficient method for drug design may enable customers to accelerate the pace of research for debilitating disorders like Alzheimer's, Parkinson's, and Huntington's diseases, as well as research for cancer and gene mutations
North America Thyroid Cancer Diagnostics Market Scope
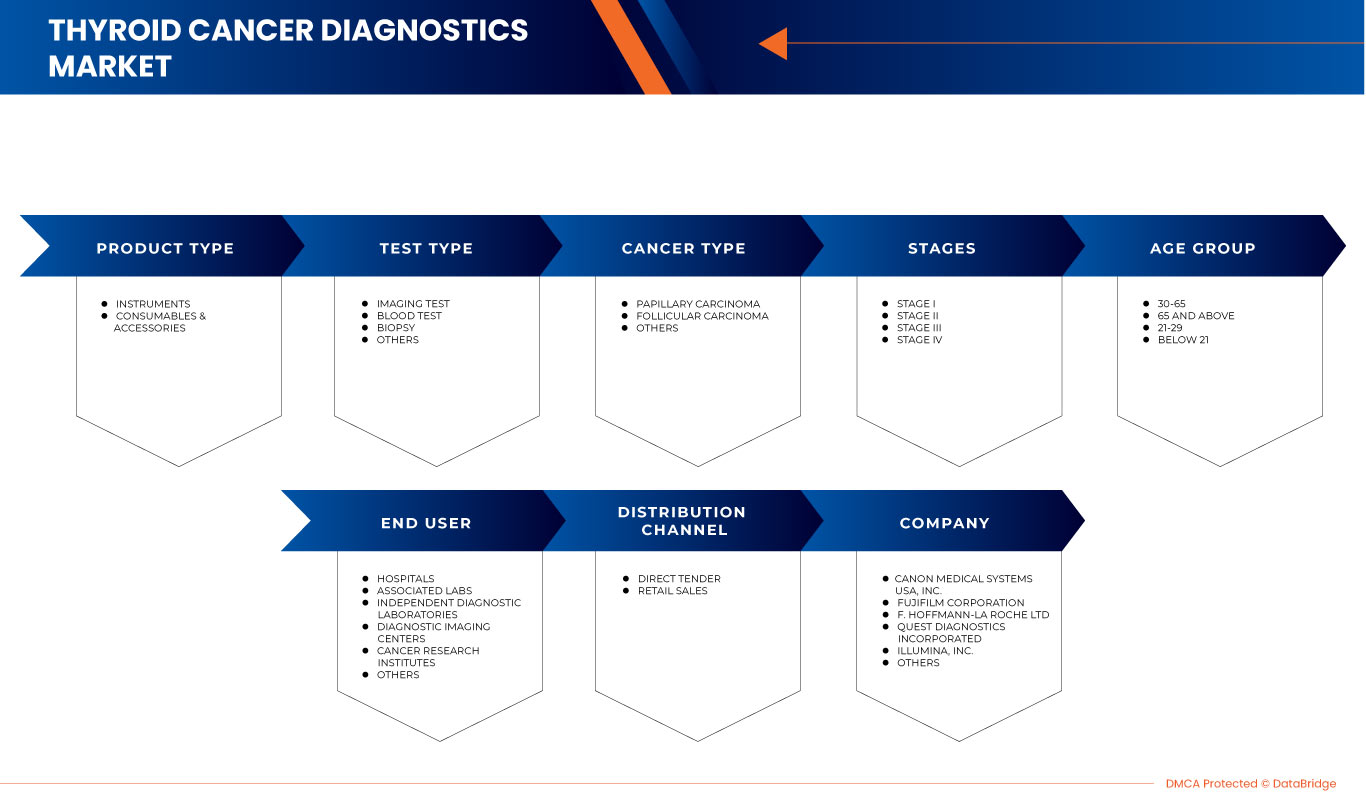
The North America thyroid cancer diagnostics market is segmented into product type, test type, cancer type, stages, age group, end user, and distribution channel. The growth amongst these segments will help you analyze meager growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
Product Type
- Instruments
- Consumables & Accessories
On the basis of product type, the North America thyroid cancer diagnostics market is segmented into instruments and consumables & accessories.
Test Type
- Imaging Test
- Blood Test
- Biopsy
- Others
On the basis of test type, the North America thyroid cancer diagnostics market is segmented into imaging test, blood test, biopsy and others.
Cancer Type
- Papillary Carcinoma
- Follicular Carcinoma
- Others
On the basis of cancer type, the North America thyroid cancer diagnostics market is segmented into papillary carcinoma, follicular carcinoma, and others.
Stages
- Stage I
- Stage II
- Stage III
- Stage IV
On the basis of stages, the North America thyroid cancer diagnostics market is segmented into stage I, stage II, stage III and stage IV.
Age Group
- 30-65
- 65 and above
- 21-29
- Below 21
On the basis of age group, the North America thyroid cancer diagnostics market is segmented into 30-65, 65 and above, 21-29, and below 21.
End User
- Hospitals
- Associated Labs
- Independent Diagnostic Laboratories
- Diagnostic Imaging Centers
- Cancer Research Institutes
- Others
On the basis of end user, the North America thyroid cancer diagnostics market is segmented into hospitals, associated labs, independent diagnostic laboratories, diagnostic imaging centers, cancer research institutes, and others.
Distribution Channel
- Direct Tender
- Retail Sales
On the basis of distribution channel, the North America thyroid cancer diagnostics market is segmented into direct tender and retail sales.
North America Thyroid Cancer Diagnostics Market Regional Analysis/Insights
The North America thyroid cancer diagnostics market is analysed, and market size insights and trends are provided by country, product type, test type, cancer type, stages, age group, end user, and distribution channel, as referenced above.
The countries covered in this market report are U.S., Canada, and Mexico.
The U.S. is expected to dominate the North America thyroid cancer diagnostics market in terms of market share and revenue and will continue to flourish its dominance during the forecast period. This is due to rising thyroid cancer diagnostic tests.
The country section of the report also provides individual market-impacting factors and changes in regulations in the market that impact the current and future trends of the market. Data points, such as new and replacement sales, country demographics, disease epidemiology, and import-export tariffs, are some of the major pointers used to forecast the market scenario for individual countries. In addition, the presence and availability of North American brands and the challenges faced due to competition from local and domestic brands and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and North America Thyroid Cancer Diagnostics Market Share Analysis
North America thyroid cancer diagnostics market competitive landscape provides details by the competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, North America presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to the North America thyroid cancer diagnostics market.
北米の甲状腺がん診断市場で活動している主要企業としては、キヤノン株式会社、富士フイルムホールディングス株式会社、F. ホフマン・ラ・ロシュ株式会社、クエスト・ダイアグノスティクス社、イルミナ、フィリップス社、サーモフィッシャーサイエンティフィック株式会社、シーメンスヘルスケア社、アボット社、ゼネラル・エレクトリック社、BD、キアゲン社、ディアソリン社、メルク社、ホロジック社、ミリアド・ジェネティクス社、バイオメリュー社、FONAR社、タイム・メディカル・ホールディングス社、プレックスバイオ社、ミンファウンド・メディカル・システムズ社、メドニカ社、北京O&Dバイオテック社、スターンメッド社などがあります。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET TESTING TYPE COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
4.3 GROWTH STRATEGIES ADOPTED BY KEY MARKET PLAYERS
5 EPIDEMIOLOGY
6 REGULATORY FRAMEWORK OF THE NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET
6.1 REGULATORY SCENARIO IN THE U.S.
6.2 REGULATORY SCENARIO IN AUSTRALIA
6.3 REGULATORY SCENARIO IN JAPAN
6.4 REGULATORY SCENARIO IN CHINA
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 RISING INCIDENCE AND PREVALENCE OF THYROID NODULES AND CANCER
7.1.2 RISING THYROID CANCER DIAGNOSTIC TESTS
7.1.3 RISING PREFERENCE FOR PREVENTIVE HEALTH CHECK-UPS
7.1.4 RISING AWARENESS TOWARDS THYROID CANCER
7.2 RESTRAINTS
7.2.1 HIGH COST OF DIAGNOSTICS PROCEDURE
7.2.2 TISSUE DAMAGE DUE TO HIGH RADIATION EXPOSURE FROM IMAGING TESTS
7.3 OPPORTUNITIES
7.3.1 RISE IN HEALTHCARE EXPENDITURE FOR CANCER DIAGNOSIS AND TREATMENT
7.3.2 RISING OBESE POPULATION
7.4 CHALLENGES
7.4.1 STRINGENT REGULATORY FRAMEWORK FOR THE APPROVAL AND COMMERCIALIZATION OF CANCER DIAGNOSTIC PRODUCTS
7.4.2 LACK OF SKILLED AND CERTIFIED EXPERTISE
8 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE
8.1 OVERVIEW
8.2 INSTRUMENTS
8.2.1 PATHOLOGY BASED INSTRUMENTS
8.2.1.1 PCR INSTRUMENTS
8.2.1.2 SLIDE STAINING SYSTEMS
8.2.1.3 TISSUE PROCESSING SYSTEMS
8.2.1.4 CELL PROCESSORS
8.2.1.5 OTHER PATHOLOGY-BASED INSTRUMENTS
8.2.2 IMAGING INSTRUMENTS
8.2.2.1 ULTRASOUND SYSTEMS
8.2.2.2 CT SYSTEMS
8.2.2.3 MRI SYSTEMS
8.2.2.4 OTHERS
8.2.3 BIOPSY INSTRUMENTS
8.2.3.1 NEEDLE BIOPSY
8.2.3.2 ENDOSCOPIC BIOPSY
8.2.3.3 CORE BIOPSY
8.2.3.4 OTHERS
8.2.4 OTHERS
8.3 CONSUMABLES & ACCESSORIES
8.3.1 KITS
8.3.1.1 PCR KITS
8.3.1.2 DNA POLYMERASE KITS
8.3.1.3 NUCLEIC ACID ISOLATION KITS
8.3.1.4 OTHERS
8.3.2 REAGENTS
8.3.2.1 ASSAYS
8.3.2.2 BUFFERS
8.3.2.3 PRIMERS
8.3.2.4 OTHERS
8.3.3 PROBES
8.3.4 OTHER CONSUMABLES
9 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE
9.1 OVERVIEW
9.2 IMAGING TEST
9.2.1 COMPUTED TOMOGRAPHY (CT) SCAN
9.2.2 MRI
9.2.3 POSITRON EMISSION TOMOGRAPHY (PET) SCAN
9.2.4 OTHERS
9.3 BLOOD TEST
9.3.1 BLOOD CHEMISTRY TESTS
9.3.2 COMPLETE BLOOD COUNT (CBC)
9.3.3 OTHERS
9.4 BIOPSY
9.4.1 NEEDLE BIOPSY
9.4.2 BRONCHOSCOPY BIOPSY
9.4.3 CORE BIOPSY
9.4.4 OTHERS
9.5 OTHERS
10 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY CANCER TYPE
10.1 OVERVIEW
10.2 PAPILLARY CARCINOMA
10.3 FOLLICULAR CARCINOMA
10.4 OTHERS
11 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY STAGES
11.1 OVERVIEW
11.2 STAGE I
11.3 STAGE II
11.4 STAGE III
11.5 STAGE IV
12 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY AGE GROUP
12.1 OVERVIEW
12.2 30-65
12.3 65 AND ABOVE
12.4 21-29
12.5 BELOW 21
13 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY END USER
13.1 OVERVIEW
13.2 HOSPITALS
13.3 ASSOCIATED LABS
13.4 INDEPENDENT DIAGNOSTIC LABORATORIES
13.5 DIAGNOSTIC IMAGING CENTERS
13.6 CANCER RESEARCH INSTITUTES
13.7 OTHERS
14 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
14.1 OVERVIEW
14.2 DIRECT TENDER
14.3 RETAIL SALES
15 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY REGION
15.1 NORTH AMERICA
15.1.1 U.S.
15.1.2 CANADA
15.1.3 MEXICO
16 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
17 SWOT ANALYSIS
18 COMPANY PROFILE
18.1 CANON INC.
18.1.1 COMPANY SNAPSHOT
18.1.2 COMPANY SHARE ANALYSIS
18.1.3 PRODUCT PORTFOLIO
18.1.4 RECENT DEVELOPMENTS
18.2 FUJIFILM CORPORATION
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY SHARE ANALYSIS
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENT
18.3 F. HOFFMANN-LA ROCHE LTD
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 COMPANY SHARE ANALYSIS
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPMENT
18.4 QUEST DIAGNOSTICS INCORPORATED
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 COMPANY SHARE ANALYSIS
18.4.4 PRODUCT PORTFOLIO
18.4.5 RECENT DEVELOPMENTS
18.5 ILLUMINA, INC.
18.5.1 COMPANY SNAPSHOT
18.5.2 REVENUE ANALYSIS
18.5.3 COMPANY SHARE ANALYSIS
18.5.4 PRODUCT PORTFOLIO
18.5.5 RECENT DEVELOPMENT
18.6 ABBOTT
18.6.1 COMPANY SNAPSHOT
18.6.2 REVENUE ANALYSIS
18.6.3 PRODUCT PORTFOLIO
18.6.4 RECENT DEVELOPMENT
18.7 BD
18.7.1 COMPANY SNAPSHOT
18.7.2 REVENUE ANALYSIS
18.7.3 PRODUCT PORTFOLIO
18.7.4 RECENT DEVELOPMENT
18.8 BEIJING O&D BIOTECH CO., LTD.
18.8.1 COMPANY SNAPSHOT
18.8.2 PRODUCT PORTFOLIO
18.8.3 RECENT DEVELOPMENTS
18.9 BIOMÉRIEUX SA
18.9.1 COMPANY SNAPSHOT
18.9.2 REVENUE ANALYSIS
18.9.3 PRODUCT PORTFOLIO
18.9.4 RECENT DEVELOPMENTS
18.1 DIASORIN S.P.A.
18.10.1 COMPANY SNAPSHOT
18.10.2 REVENUE ANALYSIS
18.10.3 PRODUCT PORTFOLIO
18.10.4 RECENT DEVELOPMENT
18.11 FONAR CORP.
18.11.1 COMPANY SNAPSHOT
18.11.2 REVENUE ANALYSIS
18.11.3 PRODUCT PORTFOLIO
18.11.4 RECENT DEVELOPMENTS
18.12 GENERAL ELECTRIC
18.12.1 COMPANY SNAPSHOT
18.12.2 REVENUE ANALYSIS
18.12.3 PRODUCT PORTFOLIO
18.12.4 RECENT DEVELOPMENTS
18.13 HOLOGIC INC.
18.13.1 COMPANY SNAPSHOT
18.13.2 REVENUE ANALYSIS
18.13.3 PRODUCT PORTFOLIO
18.13.4 RECENT DEVELOPMENT
18.14 KONINKLIJKE PHILIPS N.V.
18.14.1 COMPANY SNAPSHOT
18.14.2 REVENUE ANALYSIS
18.14.3 PRODUCT PORTFOLIO
18.14.4 RECENT DEVELOPMENT
18.15 MERCK KGAA.
18.15.1 COMPANY SNAPSHOT
18.15.2 REVENUE ANALYSIS
18.15.3 PRODUCT PORTFOLIO
18.15.4 RECENT DEVELOPMENT
18.16 MEDONICA CO. LTD
18.16.1 COMPANY SNAPSHOT
18.16.2 PRODUCT PORTFOLIO
18.16.3 RECENT DEVELOPMENT
18.17 MINFOUND MEDICAL SYSTEMS CO. LTD
18.17.1 COMPANY SNAPSHOT
18.17.2 PRODUCT PORTFOLIO
18.17.3 RECENT DEVELOPMENTS
18.18 MYRIAD GENETICS, INC.
18.18.1 COMPANY SNAPSHOT
18.18.2 REVENUE ANALYSIS
18.18.3 PRODUCT PORTFOLIO
18.18.4 RECENT DEVELOPMENTS
18.19 PLEXBIO.
18.19.1 COMPANY SNAPSHOT
18.19.2 PRODUCT PORTFOLIO
18.19.3 RECENT DEVELOPMENTS
18.2 QIAGEN
18.20.1 COMPANY SNAPSHOT
18.20.2 REVENUE ANALYSIS
18.20.3 PRODUCT PORTFOLIO
18.20.4 RECENT DEVELOPMENT
18.21 STERNMED GMBH
18.21.1 COMPANY SNAPSHOT
18.21.2 PRODUCT PORTFOLIO
18.21.3 RECENT DEVELOPMENTS
18.22 SIEMENS HEALTHCARE GMBH
18.22.1 COMPANY SNAPSHOT
18.22.2 REVENUE ANALYSIS
18.22.3 PRODUCT PORTFOLIO
18.22.4 RECENT DEVELOPMENT
18.23 TIME MEDICAL HOLDING.
18.23.1 COMPANY SNAPSHOT
18.23.2 PRODUCT PORTFOLIO
18.23.3 RECENT DEVELOPMENT
18.24 THERMO FISHER SCIENTIFIC INC.
18.24.1 COMPANY SNAPSHOT
18.24.2 REVENUE ANALYSIS
18.24.3 PRODUCT PORTFOLIO
18.24.4 RECENT DEVELOPMENT
19 QUESTIONNAIRE
20 RELATED REPORTS
表のリスト
TABLE 1 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 2 NORTH AMERICA INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 3 NORTH AMERICA INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA PATHOLOGY-BASED INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA IMAGING INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA BIOPSY INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA CONSUMABLES & ACCESSORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA CONSUMABLES & ACCESSORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA KITS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA REAGENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA IMAGING TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA IMAGING TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA BLOOD TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA BLOOD TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA BIOPSY IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA BIOPSY IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA OTHERS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA PAPILLARY CARCINOMA IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA FOLLICULAR CARCINOMA IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA OTHERS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA STAGE I IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 25 NORTH AMERICA STAGE II IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 NORTH AMERICA STAGE III IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 27 NORTH AMERICA STAGE IV IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 28 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 29 NORTH AMERICA 30-65 IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 NORTH AMERICA 65 AND ABOVE IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 31 NORTH AMERICA 21-29 IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 NORTH AMERICA BELOW 21 IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 33 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 34 NORTH AMERICA HOSPITALS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 35 NORTH AMERICA ASSOCIATED LABS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 36 NORTH AMERICA INDEPENDENT DIAGNOSTIC LABORATORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 37 NORTH AMERICA DIAGNOSTIC IMAGING CENTERS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 38 NORTH AMERICA CANCER RESEARCH INSTITUTES IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 39 NORTH AMERICA OTHERS IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 40 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 41 NORTH AMERICA DIRECT TENDER IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 42 NORTH AMERICA RETAIL SALES IN THYROID CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 43 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 44 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 45 NORTH AMERICA INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 46 NORTH AMERICA PATHOLOGY-BASED INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 47 NORTH AMERICA IMAGING INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 48 NORTH AMERICA BIOPSY INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 49 NORTH AMERICA CONSUMABLES & ACCESSORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 50 NORTH AMERICA KITS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 51 NORTH AMERICA REAGENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 52 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 53 NORTH AMERICA IMAGING TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 54 NORTH AMERICA BIOPSY IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 55 NORTH AMERICA BLOOD TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 56 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 57 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 58 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 59 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 60 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 61 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 62 U.S. INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 63 U.S. PATHOLOGY-BASED INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 64 U.S. IMAGING INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 65 U.S. BIOPSY INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 66 U.S. CONSUMABLES & ACCESSORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 67 U.S. KITS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 68 U.S. REAGENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 69 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 70 U.S. IMAGING TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 71 U.S. BIOPSY IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 72 U.S. BLOOD TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 73 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 74 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 75 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 76 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 77 U.S. THYROID CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 78 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 79 CANADA INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 80 CANADA PATHOLOGY-BASED INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 81 CANADA IMAGING INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 82 CANADA BIOPSY INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 83 CANADA CONSUMABLES & ACCESSORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 84 CANADA KITS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 85 CANADA REAGENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 86 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 87 CANADA IMAGING TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 88 CANADA BIOPSY IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 89 CANADA BLOOD TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 90 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 91 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 92 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 93 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 94 CANADA THYROID CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 95 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 96 MEXICO INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 97 MEXICO PATHOLOGY-BASED INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 98 MEXICO IMAGING INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 99 MEXICO BIOPSY INSTRUMENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 100 MEXICO CONSUMABLES & ACCESSORIES IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 101 MEXICO KITS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 102 MEXICO REAGENTS IN THYROID CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 103 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 104 MEXICO IMAGING TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 105 MEXICO BIOPSY IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 106 MEXICO BLOOD TEST IN THYROID CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 107 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 108 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 109 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 110 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 111 MEXICO THYROID CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
図表一覧
FIGURE 1 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: MARKET TESTING TYPE COVERAGE GRID
FIGURE 9 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 11 THE INCREASE IN THE AWARENESS ABOUT THYROID CANCER IS EXPECTED TO DRIVE THE NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET IN THE FORECAST PERIOD
FIGURE 12 PRODUCT TYPE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET
FIGURE 14 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, 2022
FIGURE 15 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, 2023-2030 (USD MILLION)
FIGURE 16 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, CAGR (2023-2030)
FIGURE 17 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 18 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY TEST TYPE, 2022
FIGURE 19 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY TEST TYPE, 2023-2030 (USD MILLION)
FIGURE 20 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY TEST TYPE, CAGR (2023-2030)
FIGURE 21 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 22 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, 2022
FIGURE 23 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, 2023-2030 (USD MILLION)
FIGURE 24 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, CAGR (2023-2030)
FIGURE 25 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, LIFELINE CURVE
FIGURE 26 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY STAGES, 2022
FIGURE 27 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY STAGES, 2023-2030 (USD MILLION)
FIGURE 28 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY STAGES, CAGR (2023-2030)
FIGURE 29 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY STAGES, LIFELINE CURVE
FIGURE 30 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY AGE GROUP, 2022
FIGURE 31 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY AGE GROUP, 2023-2030 (USD MILLION)
FIGURE 32 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY AGE GROUP, CAGR (2023-2030)
FIGURE 33 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY AGE GROUP, LIFELINE CURVE
FIGURE 34 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY END USER, 2022
FIGURE 35 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 36 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 37 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY END USER, LIFELINE CURVE
FIGURE 38 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 39 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 40 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 41 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 42 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 43 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 44 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 45 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 46 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: PRODUCT TYPE (2023-2030)
FIGURE 47 NORTH AMERICA THYROID CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。