Global Autonomous Medical Device Market
시장 규모 (USD 10억)
연평균 성장률 :
% 
 USD
672.42 Million
USD
1,425.14 Million
2024
2032
USD
672.42 Million
USD
1,425.14 Million
2024
2032
| 2025 –2032 | |
| USD 672.42 Million | |
| USD 1,425.14 Million | |
|
|
|
|
Global Autonomous Medical Device Market Segmentation, By Product Type (Robotic Surgery Devices, Autonomous Diagnostic Devices, Wearable Autonomous Medical Devices, Therapeutic Autonomous Devices, and Monitoring Devices), Technology (Artificial Intelligence (AI), Machine Learning, Internet of Medical Things (IoMT), Robotics, and Blockchain Technology), Application (Surgery, Diagnostics, Therapeutics, Patient Monitoring, and Rehabilitation), End-User (Hospitals, Clinics, Home Care, and Research and Academic Institutions) – Industry Trends and Forecast to 2032
Autonomous Medical Device Market Analysis
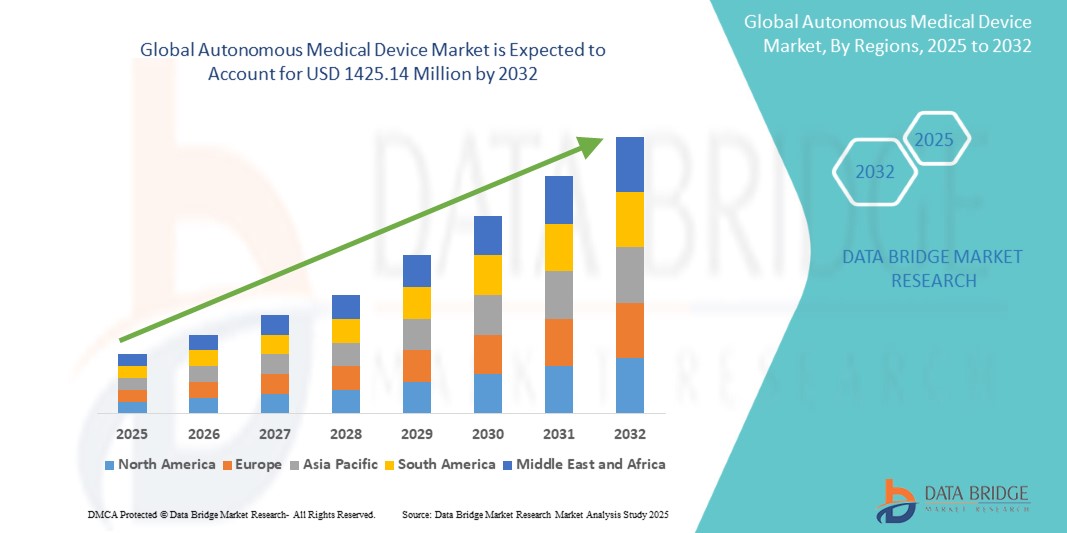
The global autonomous medical device market is expanding due to the rising prevalence of chronic diseases and the growing demand for continuous monitoring and treatment solutions. With over 450 million people worldwide affected by diabetes and 1.28 billion adults suffering from hypertension, the need for autonomous devices such as insulin pumps and wearable monitors is increasing. Additionally, the aging population, which surpassed 1.4 billion in 2020, drives the demand for autonomous solutions in elderly care. Technological advancements in AI, robotics, and machine learning are also improving surgical precision and patient outcomes, further fueling market growth.
Autonomous Medical Device Market Size
Global autonomous medical device market size was valued at USD 672.42 million in 2024 and is projected to reach USD 1425.14 million by 2032, with a CAGR of 11.50% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Autonomous Medical Device Market Trends
“Focus on Elderly Care”
A prominent trend in the global Autonomous Medical Device market is the increasing focus on elderly care, spurred by the growing aging population. As the number of older adults continues to rise, there is a noticeable shift toward the development of autonomous devices designed to support independent living and improve quality of life for the elderly. Devices such as fall detection systems, which can alert caregivers in case of an accident, and automated medication dispensers, which ensure timely and accurate delivery of prescribed drugs, are becoming more prevalent in both home care settings and long-term care facilities. Health monitoring tools, such as wearable sensors that track vital signs or detect early signs of health issues, are also gaining traction. These devices offer real-time data, providing both patients and caregivers with actionable insights, thus enhancing overall care management and safety. This trend reflects the growing need for solutions that enable elderly individuals to live more independently while maintaining health and safety.
Report Scope and Autonomous Medical Device Market Segmentation
|
Attributes |
Autonomous Medical Device Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa, Brazil, Argentina, and Rest of South America |
|
Key Market Players |
Intuitive Surgical, Inc. (U.S.), Medtronic (Ireland), Johnson & Johnson Services, Inc. (U.S.), Stryker (U.S.), Siemens Healthineers (Germany), GE Healthcare (U.S.), Abbott (U.S.), Koninklijke Philips N.V. (Netherlands), Boston Scientific Corporation (U.S.), Zimmer Biomet (U.S.), Aethon, Inc. (U.S.), iRobot Corporation (U.S.), Medrobotics Corporation (U.S.), Livanova (UK), Endo International PLC (Ireland), Xenex Disinfection Services, Inc. (U.S.), Omron Healthcare, Inc. (Japan), and NeuroPace, Inc. (Inc.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Autonomous Medical Device Market Definition
The global autonomous medical device market refers to the sector involving medical devices that operate independently, using advanced technologies such as artificial intelligence (AI), machine learning, robotics, and sensor systems to perform healthcare tasks with minimal human intervention. These devices are designed to autonomously monitor, diagnose, treat, or assist patients, often in real-time, providing solutions in areas such as surgery, diagnostics, therapeutic treatments, and chronic disease management. They include robotic surgical systems, AI-powered diagnostic tools, wearable health monitoring devices, and automated drug delivery systems, among others. The market is driven by advancements in technology, increased demand for remote healthcare, and the growing need for efficient, accurate, and personalized medical solutions.
Autonomous Medical Device Market Dynamics
Drivers
- Increasing Technological Advancements
Technological advancements, particularly in artificial intelligence (AI), machine learning, and robotics, are significantly driving the adoption of autonomous medical devices. AI and machine learning enable these devices to analyze vast amounts of healthcare data, improving the accuracy of diagnostics and treatment plans. For instance, AI-powered systems can interpret medical images, detect patterns, and predict health outcomes with high precision, reducing human error and enhancing early diagnosis. Robotics, on the other hand, enables highly precise surgical procedures, allowing for minimally invasive operations and faster recovery times. The integration of these technologies in patient monitoring devices further enhances their effectiveness by providing real-time data analysis and alerts, ensuring timely medical intervention. Additionally, automation in drug delivery systems and remote healthcare solutions, such as telemedicine platforms, benefit from these technological advancements, offering personalized care while improving overall healthcare efficiency. These innovations are transforming medical practices and are pivotal in the growth of the autonomous medical device market. For instance, in August 2024, the FDA has approved 950 AI or machine learning-enabled devices, with a significant rise in submissions in recent years. This surge in AI integration is expected to drive the global autonomous medical device market, aligning with the trend of "increasing technological advancements.
- Rising Aging Population
The global aging population is a key driver of the demand for autonomous medical devices. As the number of elderly individuals continues to rise, particularly in regions such as North America, Europe, and Asia-Pacific, the need for effective healthcare solutions tailored to this demographic is growing. Older adults often face chronic conditions such as diabetes, hypertension, and cardiovascular diseases, which require constant monitoring and management. Autonomous medical devices, including wearable health monitors, automated medication dispensers, and remote monitoring systems, are increasingly being used to address these needs. These devices provide real-time data, allowing healthcare providers to track patients' health remotely, ensuring timely interventions and reducing the need for frequent hospital visits. Additionally, autonomous solutions such as fall detection systems and robotic care assistants enhance safety and support elderly individuals in living independently for longer periods. The aging population is, therefore, a critical factor fueling the demand for innovative and reliable autonomous medical devices. In 2022, the population aged 65 and above is growing faster than those below that age. This demographic shift is expected to drive the global autonomous medical device market, as the increasing elderly population demands more healthcare solutions, justifying the "rising aging population" trend.
Opportunities
- Expansion in Remote Healthcare Solutions
The expansion of remote healthcare solutions presents a significant opportunity for the global autonomous medical device market. As telemedicine and remote patient monitoring gain traction, particularly in rural or underserved regions, autonomous medical devices are poised to bridge gaps in healthcare access. These devices, including wearable health monitors, remote diagnostic tools, and telehealth platforms, enable real-time health tracking and virtual consultations, allowing patients to receive care without the need for frequent in-person visits. This is particularly beneficial for individuals with chronic conditions who require continuous monitoring, such as those with diabetes, cardiovascular diseases, or respiratory issues. Autonomous devices can transmit health data directly to healthcare providers, enabling timely interventions and reducing hospital admissions. By offering cost-effective and accessible healthcare solutions, these devices enhance patient convenience, improve care outcomes, and address the growing demand for healthcare services in remote and resource-limited areas, thus expanding the market’s potential.
- Growth in Personalized Healthcare
The growth in personalized healthcare presents a valuable opportunity for the Global Autonomous Medical Device Market. Autonomous medical devices powered by AI and machine learning can analyze vast amounts of individual health data to deliver tailored treatments and interventions. These devices use data from wearable sensors, genetic information, and lifestyle factors to create personalized treatment plans, improving the effectiveness of care. For instance, AI-driven devices can optimize medication dosages, monitor real-time health conditions, and adjust therapies based on the patient's specific needs. This personalized approach is especially beneficial in managing chronic diseases such as diabetes, hypertension, and cardiovascular conditions, where ongoing adjustments and precise monitoring are crucial. By offering more accurate, individualized care, autonomous medical devices not only enhance patient outcomes but also streamline chronic disease management, reducing hospital visits and overall healthcare costs. This trend toward personalized care is driving the demand for advanced, autonomous healthcare solutions.
Restraints/Challenges
- High Cost of Development and Implementation of Autonomous Medical Devices
The high cost of development and implementation is a key restraint in the global autonomous medical device market. Creating autonomous medical devices requires substantial investments in advanced technologies such as artificial intelligence (AI), machine learning, and robotics, which are essential for their functionality and accuracy. Additionally, these devices need to undergo rigorous clinical trials and secure regulatory approvals from various health authorities, which involve both time and significant financial resources. The cost of production and compliance with regulatory standards can make these devices expensive, limiting their affordability and accessibility. This is particularly challenging in low-income regions and smaller healthcare facilities, which may struggle to afford or adopt these high-tech solutions. As a result, the high upfront costs can slow down the widespread adoption of autonomous medical devices, restricting their potential market growth and limiting their availability to certain demographics or healthcare settings. The Titanium Autonomous Medical Device is priced at USD 165.26, as per IndiaMART InterMESH Ltd. This high cost reflects the advanced materials and precision involved, underscoring the "High Cost of Advanced Instruments" as a restraint for the global autonomous medical device market. Such premium pricing limits accessibility, especially in low-income regions and smaller healthcare facilities, where budget constraints hinder the adoption of these advanced tools, thereby slowing market growth.
- Regulatory and Compliance Issues
Regulatory and compliance issues present a significant challenge for the global autonomous medical device market. These devices must meet stringent safety, efficacy, and privacy requirements set by health authorities such as the FDA, EMA, and other regulatory bodies worldwide. The process of obtaining approval is complex and time-consuming, involving extensive clinical trials, documentation, and ongoing testing to demonstrate the device's reliability and safety. In addition, the regulatory frameworks vary significantly across countries, with each region having its own set of rules and standards for medical devices. This creates barriers for manufacturers trying to introduce products to multiple markets, as they must navigate different regulatory landscapes, which can delay product launches and increase costs. Furthermore, ensuring compliance with data privacy laws, especially regarding patient information, adds another layer of complexity. These factors can impede the speed at which autonomous medical devices are brought to market, hindering adoption and growth. In April 2024, according to an article published by tmc.gov.in, instruments must hold a European CE marking or USFDA certification, be manufactured by companies with over 30 years of experience, and have a chromium content of at least 12% to ensure adequate corrosion resistance. These stringent requirements highlight the "Stringent Regulatory Standards" in the global autonomous medical device market. Such high standards increase manufacturing costs and extend approval timelines, posing challenges for smaller manufacturers and delaying market entry. This regulatory burden can hinder the adoption of advanced Autonomous Medical Devices, especially in regions with less stringent regulations, ultimately limiting market growth
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Autonomous Medical Device Market Scope
The market is segmented on the basis of product type, technology, application, and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product Type
- Robotic Surgery Devices
- Autonomous Diagnostic Devices
- Wearable Autonomous Medical Devices
- Therapeutic Autonomous Devices
- Monitoring Devices
Technology
- Artificial Intelligence (AI)
- Machine Learning
- Internet of Medical Things (IoMT)
- Robotics
- Blockchain Technology
Application
- Surgery
- Diagnostics
- Therapeutics
- Patient Monitoring
- Rehabilitation
End-User
- Hospitals
- Clinics
- Home Care
- Research and Academic Institutions
Autonomous Medical Device Market Regional Analysis
The market is analyzed and market size insights and trends are provided by country, product type, technology, application, and end-user as referenced above.
The countries covered in the market are U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, rest of Middle East and Africa, Brazil, Argentina, and rest of South America.
North America is expected to dominate the market due to its well-established healthcare infrastructure, which ensures the availability of state-of-the-art medical facilities and highly skilled professionals. The region also has a high adoption rate of advanced technologies, including minimally invasive surgical techniques and innovative graft materials, which improve patient outcomes.
Asia-Pacific is expected to be the fastest growing due to rapidly improving healthcare infrastructure, increasing adoption of advanced technologies, and a large aging population requiring innovative medical solutions.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Autonomous Medical Device Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Autonomous Medical Device Market Leaders Operating in the Market Are:
- Intuitive Surgical, Inc. (U.S.)
- Medtronic (Ireland)
- Johnson & Johnson Services, Inc. (U.S.)
- Stryker (U.S.)
- Siemens Healthineers (Germany)
- GE Healthcare (U.S.)
- Abbott (U.S.)
- Koninklijke Philips N.V. (Netherlands)
- Boston Scientific Corporation (U.S.)
- Zimmer Biomet (U.S.)
- Aethon, Inc. (U.S.)
- iRobot Corporation (U.S.)
- Medrobotics Corporation (U.S.)
- Livanova (UK)
- Endo International PLC (Ireland)
- Xenex Disinfection Services, Inc. (U.S.)
- Omron Healthcare, Inc. (Japan)
- NeuroPace, Inc. (Inc.)
Latest Developments in Autonomous Medical Device Market
- In November 2023, Surtex Instruments introduced its "Infinex" series at MEDICA 2023, offering microsurgery instruments designed to give surgeons exceptional control and precision, even in the most intricate procedures. This innovation will help the company strengthen its position in the surgical instruments market by meeting the growing demand for high-performance tools, which are crucial for complex surgeries. The launch is expected to enhance Surtex's reputation for quality and precision, attracting more healthcare professionals and expanding its market share in the global Autonomous Medical Device industry.
- In October 2020, Katena Products has acquired Micro-Select Instruments, a manufacturer of ophthalmic instruments such as forceps, Autonomous Medical Devices, and speculums. This acquisition strengthens Katena’s instrument manufacturing capabilities and expands its product portfolio, enhancing its position in the ophthalmic surgical market. The move is expected to broaden Katena’s reach and offer a more comprehensive range of high-quality instruments to healthcare professionals, driving further growth in the global Autonomous Medical Device market.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.