North America Pcr Devices Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
9.30 Billion
USD
28.54 Billion
2024
2032
USD
9.30 Billion
USD
28.54 Billion
2024
2032
| 2025 –2032 | |
| USD 9.30 Billion | |
| USD 28.54 Billion | |
|
|
|
|
North America Polymerase Chain Reaction (PCR) Devices Market Segmentation, By Technology (Digital Polymerase Chain Reaction (PCR) and Real-time Polymerase Chain Reaction (PCR)), Product Type (Instrument, Reagent, Consumables and Others), End User (Hospital, Diagnostic Centre, Pharmaceutical and Biotechnology Companies, Clinical Research Organizations, Academia and Laboratories), Application (Oncology, Blood Testing, Pathogen Detection, Research, Forensic and Others)- Industry Trends and Forecast to 2032
Polymerase Chain Reaction (PCR) Devices Market Size
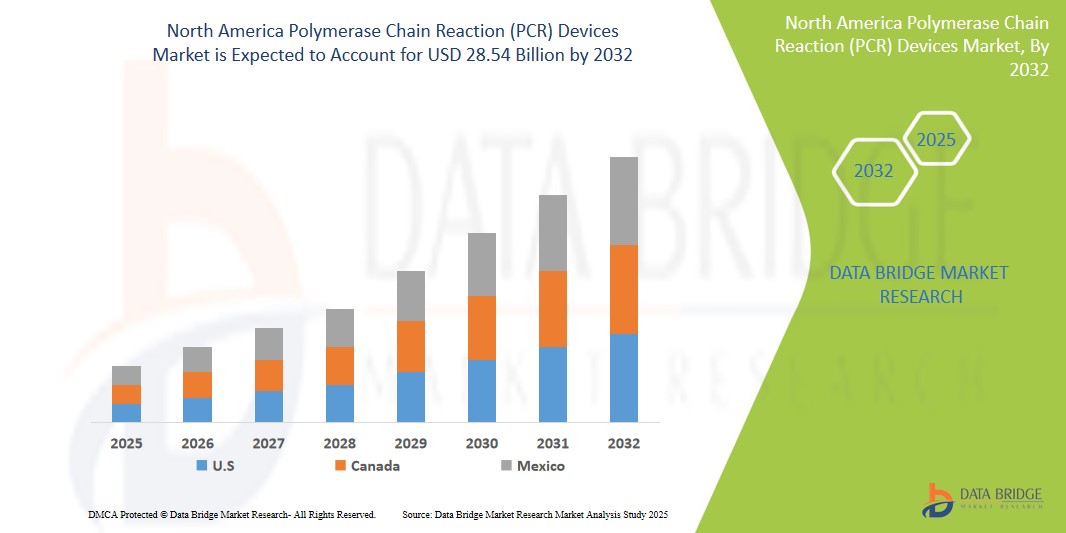
- The North America Polymerase Chain Reaction (PCR) Devices Market was valued atUSD9.3 Billion in 2024 and is expected to reachUSD28.54 Billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at aCAGR of 8.1%primarily driven by the anticipated launch of therapies
- The drivers of the Polymerase Chain Reaction (PCR) Devices Market include the growing demand for safe and effective diagnostic solutions, technological advancements in PCR devices, and the increasing prevalence of genetic disorders and infectious diseases, which require advanced diagnostic techniques.
North America Polymerase Chain Reaction (PCR) Devices Market Analysis
- Polymerase Chain Reaction (PCR) Devices play a pivotal role in molecular biology and diagnostics by amplifying specific segments of DNA or RNA for detection, analysis, and research. These devices are essential in various applications such as genetic research, medical diagnostics, forensic analysis, and environmental monitoring.
- The demand for PCR devices in North America is primarily driven by the rising prevalence of infectious diseases, advancements in molecular diagnostics, and increasing research in genetics and genomics. PCR technology is widely used in medical diagnostics, particularly for detecting genetic disorders, infectious diseases (e.g., COVID-19, HIV), and cancer.
- North America is a leading region in the global PCR devices market, bolstered by strong healthcare infrastructure, significant investments in biotechnology and pharmaceutical research, and a high level of adoption of advanced diagnostic tools. The United States, in particular, is a dominant player in the market, given its vast healthcare ecosystem, research funding, and increasing demand for diagnostic testing.
- For instance, the U.S. witnessed an increased use of PCR devices for COVID-19 testing, alongside a growing demand for genetic testing and disease surveillance.
- The North American PCR devices market is also influenced by regulatory support, including FDA approvals for novel PCR technologies, reimbursement policies, and growing research grants from both government and private sectors. Additionally, the increasing prevalence of chronic diseases and personalized medicine is accelerating the adoption of PCR devices in clinical diagnostics
Report ScopePolymerase Chain Reaction (PCR) DevicesMarket Segmentation
|
Attributes |
Polymerase Chain Reaction (PCR) DevicesKey Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Polymerase Chain Reaction (PCR) Devices Market Trends
“Advancements in PCR Technology and Growing Application Areas”
- Real-time PCR has become the gold standard in various diagnostic and research applications due to its ability to provide quantitative data. The growing use of real-time PCR in clinical diagnostics, especially for genetic testing and cancer detection, is significantly contributing to market growth.
- With the growing trend of personalized medicine, PCR devices are being used to tailor treatments based on an individual’s genetic makeup, particularly in oncology and genetic disorders.
Polymerase Chain Reaction (PCR) Devices Market Dynamics
Driver
“High Adoption of Advanced Molecular Diagnostic Techniques”
- The North America Polymerase Chain Reaction (PCR) Devices Market is driven by the increasing reliance on molecular diagnostics for early and accurate disease detection, particularly in areas such as oncology, infectious diseases, and genetic disorders.
- Government-backed healthcare programs in the U.S. and Canada, including CDC-supported disease surveillance initiatives and NIH-funded genomic research, are accelerating the adoption of PCR technologies in clinical and research settings.
- The presence of well-established diagnostic laboratories, growing integration of PCR in point-of-care testing, and rising investments in precision medicine are collectively supporting sustained market growth.
- Moreover, the post-COVID-19 era has reinforced the importance of PCR testing in healthcare infrastructure, with continued use in respiratory virus detection, including influenza and RSV.
- For instance, According to the CDC, real-time PCR remains the gold standard for detecting various viral pathogens, contributing to its expanded use in routine diagnostic protocols.
- In February 2024, Thermo Fisher Scientific reported increased demand for its real-time PCR solutions driven by a rise in cancer screening initiatives and infectious disease monitoring in North America
- This trend is further supported by growing public health awareness, advancements in automated PCR platforms, and government efforts to enhance laboratory testing capacities across the region.
Opportunity
“Integration of PCR Devices into Decentralized and Point-of-Care Testing Models”
- The increasing shift toward decentralized healthcare models, including clinics, urgent care centers, and home-based testing, is creating significant opportunities for portable and user-friendly PCR devices in North America.
- Growing demand for rapid, accurate diagnostics in non-traditional settings such as pharmacies, workplaces, and remote areas is fueling interest in compact, automated PCR platforms that deliver real-time results.
- For instance, In January 2024, according to a report by the U.S. Food and Drug Administration (FDA), there has been a surge in emergency use authorizations (EUAs) for point-of-care molecular diagnostic tools, including PCR-based assays, for respiratory infections and other emerging pathogens
- This trend is amplified by healthcare providers' need for faster clinical decision-making, particularly in infectious disease management, cancer screening, and genetic disorder detection—fostering growing investment in mobile and cloud-connected PCR solutions across North America.
Restraint/Challenge
“High Equipment Costs and Complex Regulatory Approval Processes”
- Advanced PCR devices, especially those integrated with real-time capabilities and multiplex testing features, come with high procurement and maintenance costs. This poses a significant challenge for smaller laboratories, diagnostic clinics, and rural healthcare centers across North America.
- The stringent and time-consuming regulatory requirements imposed by agencies such as the U.S. FDA and Health Canada create barriers for manufacturers aiming to launch or upgrade PCR technologies, delaying market entry and increasing development costs.
- For instance, In October 2024, a report by the Medical Device Innovation Consortium (MDIC) highlighted that the cost of bringing a new molecular diagnostic device to market can exceed USD 100 million, with regulatory compliance accounting for a large share of this expenditure
- Consequently, these financial and procedural burdens can slow down innovation, limit accessibility in underserved areas, and create competitive pressure, especially for small and medium-sized enterprises operating in the North America PCR devices market
Polymerase Chain Reaction (PCR) Devices Market Scope
The market is segmented on the basis, product type, technology, application and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Product Type |
|
|
By Technology |
|
|
By Application |
|
|
By End User
|
|
In 2025, the Real-Time PCR Devices is projected to dominate the market with a largest share in technology segment
The real-time PCR (qPCR) devices segment is expected to dominate the North America Polymerase Chain Reaction (PCR) Devices Market in 2025, owing to its widespread use in diagnostic applications, including infectious disease detection, oncology, and genetic testing. The technology’s ability to deliver rapid, accurate, and quantifiable results makes it highly valuable in both clinical and research settings. Increasing adoption in personalized medicine, alongside strong investments in biotechnology and healthcare infrastructure across the U.S. and Canada, further supports the segment’s leadership. Additionally, advancements in automation, user-friendly software, and integration with digital platforms are enhancing the usability and appeal of real-time PCR systems.
The Diagnostic Laboratories is expected to account for the largest share during the forecast period in end user market
In 2025, hospitals and diagnostic laboratories are anticipated to hold the largest share of the North America PCR Devices Market by end user, driven by the growing need for rapid and accurate diagnostic tools in managing infectious diseases, cancer, and genetic disorders. The high patient footfall, coupled with better reimbursement structures and access to advanced diagnostic technologies, enables these institutions to invest heavily in cutting-edge PCR systems. The post-pandemic emphasis on improving diagnostic capacities and the expansion of hospital laboratory services across North America further bolster the dominance of this segment.
Polymerase Chain Reaction (PCR) Devices Market Regional Analysis
“U.S. is the Dominant Country in the Polymerase Chain Reaction (PCR) Devices Market ”
- North America leads the global Polymerase Chain Reaction (PCR) Devices market, with the United States accounting for the largest share due to its highly developed healthcare infrastructure, widespread use of molecular diagnostics, and substantial investments in life sciences research.
- The growing prevalence of infectious diseases, genetic disorders, and cancer is increasing the demand for accurate and rapid diagnostic tools, further driving PCR device adoption across hospitals, diagnostic labs, and research institutions in the U.S.
- The presence of key industry players such as Thermo Fisher Scientific, Bio-Rad Laboratories, and Agilent Technologies contributes to the region’s technological edge, offering advanced and user-friendly PCR systems.
- Favorable government funding, such as NIH research grants and public health initiatives targeting early disease detection, continues to strengthen the country’s leadership position in the market.
“Canada is Projected to Register the Highest Growth Rate”
- Canada is expected to witness the fastest growth in the North America PCR Devices market, supported by its universal healthcare model and a strong national focus on improving disease surveillance and diagnostic capabilities.
- Strategic government investments in molecular diagnostics and research infrastructure—especially in response to public health challenges such as COVID-19 and antimicrobial resistance—are enhancing the adoption of PCR technologies.
- The expansion of genomics and personalized medicine programs, particularly through initiatives like Genome Canada, is boosting demand for real-time and digital PCR solutions.
- Increased collaborations between academic research centers and biotechnology firms, combined with growing awareness of early diagnosis and preventive care, are accelerating market growth across Canadian provinces
Polymerase Chain Reaction (PCR) Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Thermo Fisher Scientific (U.S.)
- Bio-Rad Laboratories (U.S.)
- Agilent Technologies (U.S.)
- Qiagen (Germany)
- Roche (Switzerland)
- Abbott Laboratories (U.S.)
- PerkinElmer Inc. (U.S.)
- Becton Dickinson and Company (U.S.)
Latest Developments in Global Polymerase Chain Reaction (PCR) Devices Market
- In September 2023, Thermo Fisher Scientific introduced an advanced line of real-time PCR instruments, offering enhanced speed and high-throughput performance. Designed for both clinical diagnostics and research, the systems feature improved sensitivity, faster thermal cycling, and intuitive software, aiming to streamline workflows in laboratories handling large sample volumes.
- In January 2024, Bio-Rad Laboratories unveiled a new multiplex PCR system focused on improving diagnostic accuracy for infectious diseases. This system enables simultaneous detection of multiple pathogens in a single run, reducing turnaround time and reagent use. The innovation supports labs facing high testing demands and contributes to efficient disease surveillance
- In March 2024, Qiagen launched a digital PCR system tailored for precision oncology and rare mutation detection. The platform offers high sensitivity and quantification capabilities, catering to growing demand for personalized medicine. Its compact design and automated workflows are ideal for clinical labs and translational research centers.
- In February 2024, Agilent Technologies announced a new AI-powered software tool for analyzing PCR data, designed to improve result accuracy and reduce manual interpretation. The tool integrates with Agilent’s existing PCR platforms, enabling real-time analytics and predictive modeling. This innovation enhances efficiency in clinical diagnostics and life sciences research environments.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.