Global Prefilled Syringes Market
Taille du marché en milliards USD
TCAC :
% 
 USD
8.04 Billion
USD
16.63 Billion
2024
2032
USD
8.04 Billion
USD
16.63 Billion
2024
2032
| 2025 –2032 | |
| USD 8.04 Billion | |
| USD 16.63 Billion | |
|
|
|
|
Global Prefilled Syringes Market Segmentation, By Product Type (Conventional Prefilled Syringes and Safety Prefilled Syringes), Material Type (Glass Prefilled Syringesand Plastic Prefilled Syringes), Closing System (Staked Needle System, Luer Cone System, and Luer Lock System), Design (Single-Chamber Prefilled Syringes, Dual- Chamber Prefilled Syringes, and Customized Prefilled Syringes), Application (Vaccines and immunizations, Diabetes, Rheumatoid Arthritis, Anaphylaxis, Cancer, Thrombosis, Ophthalmology, and Others), End User (Hospitals, Mail Order Pharmacies, Ambulatory Surgery Centers, and Others) - Industry Trends and Forecast to 2032
Prefilled Syringes Market Size
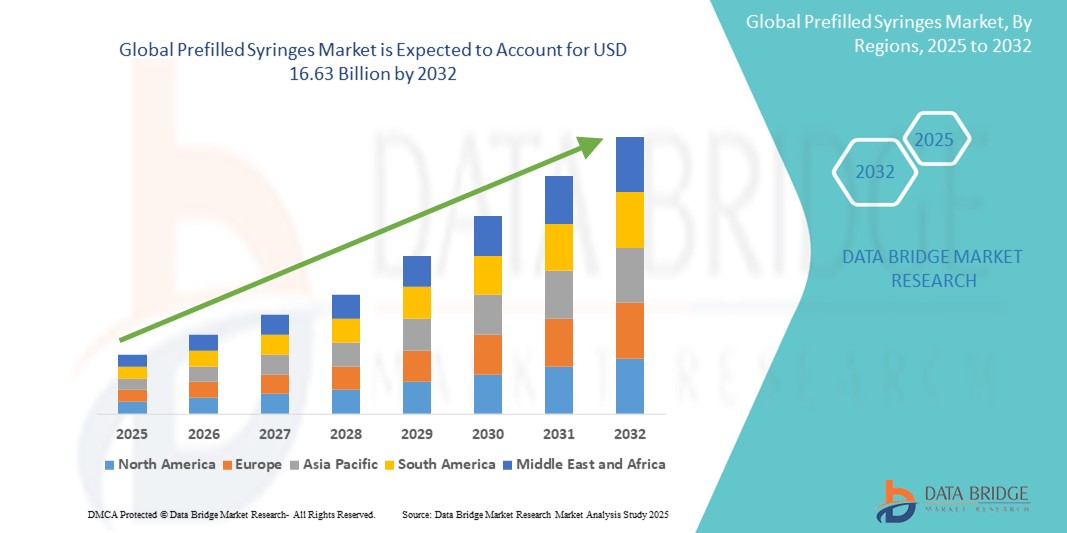
- The global prefilled syringes market size was valued atUSD 8.04 billion in 2024and is expected to reachUSD 16.63 billion by 2032, at aCAGR of 9.50%during the forecast period
- This growth is driven by factors such as the increasing demand for self-administration of injectable drugs, advancements in drug delivery technologies, and the rising prevalence of chronic diseases and biologics
Prefilled Syringes Market Analysis
- Prefilled syringes are ready-to-use injection devices that come pre-filled with a specific dosage of medication, offering convenience, safety, and reduced risk of dosing errors. They are widely used for biologics, vaccines, and chronic condition treatments
- The demand for prefilled syringes is significantly driven by the increasing adoption of biologics, self-administration of injectable drugs, and the growing prevalence of chronic diseases such as diabetes
- North America is expected to dominate the prefilled syringes market with a market share of 32.5%, due to advanced healthcare infrastructure, widespread adoption of injectable biologics, and the high prevalence of chronic diseases such as diabetes andrheumatoid arthritis
- Asia-Pacific is expected to be the fastest growing region in the prefilled syringes market with a market share of 25.3%, during the forecast period due to rapid healthcare infrastructure development, increased focus on chronic disease management, and rising healthcare awareness
- Glass prefilled syringes segment is expected to dominate the market with a market share of 50.9% due to its superior chemical resistance, excellent barrier properties, and compatibility with a wide range of drug formulations, including biologics.
Report Scope and Prefilled Syringes Market Segmentation
|
Attributes |
Prefilled Syringes Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Prefilled Syringes Market Trends
“Technological Advancements & Shift Toward Self-Administration”
- One prominent trend in the prefilled syringes market is the increasing adoption of advanced drug delivery technologies that support patient-centric care and self-administration
- These innovations include needle safety mechanisms,auto-injectors, and ergonomic syringe designs that enhance usability, reduce needlestick injuries, and improve patient compliance
- For instance, the integration of wearable injectors and connected prefilled devices enables real-time monitoring and adherence tracking, which is particularly beneficial for chronic conditions such as diabetes and rheumatoid arthritis
- These advancements are revolutionizing drug delivery by improving treatment adherence, minimizing dosing errors, and driving demand for smart, next-generation prefilled syringe solutions
Prefilled Syringes Market Dynamics
Driver
“Rising Demand Due to Chronic Disease Prevalence & Need for Safe Drug Delivery”
- The growing prevalence of chronic diseases such as diabetes, rheumatoid arthritis, and cardiovascular conditions is significantly contributing to the rising demand for prefilled syringes
- These conditions often require regular and precise administration of injectable medications, making prefilled syringes a preferred option due to their accuracy, safety, and ease of use
- As healthcare systems prioritize patient-centric treatment and home-based care, the need for reliable, ready-to-use drug delivery systems such as prefilled syringes become more pronounced
For instance,
- According to the International Diabetes Federation, approximately 537 million adults were living with diabetes globally in 2021, and this number is expected to rise to 643 million by 2030. With insulin therapy being a primary treatment, prefilled syringes play a crucial role in diabetes management
- As a result of this rising chronic disease burden and the need for efficient, safe drug administration, the demand for prefilled syringes is growing rapidly across both developed and emerging markets
Opportunity
“Emergence of Smart Prefilled Syringes and Digital Health Integration”
- The integration of smart technologies into prefilled syringes presents a significant opportunity to enhance patient adherence, monitor dosing accuracy, and support remote healthcare management
- Smart prefilled syringes equipped with connectivity features can track injection time, dosage, and frequency, transmitting data to healthcare providers or mobile health platforms in real time
- These innovations are particularly beneficial for managing chronic conditions, where consistent medication adherence is critical to successful outcomes
For instance,
- In February 2024, Ypsomed launched a connected prefilled syringe solution that enables wireless transmission of injection data to digital health apps, enhancing patient engagement and supporting personalized treatment plans. Such solutions are especially valuable for conditions such as diabetes, rheumatoid arthritis, and multiple sclerosis
- The growing demand for home-based care and the rise of digital health ecosystems create a robust opportunity for the expansion of smart prefilled syringes, ultimately improving treatment outcomes and empowering patients in managing their health more effectively
Restraint/Challenge
“Regulatory Complexities and High Manufacturing Costs”
- The stringent regulatory requirements for prefilled syringes, particularly concerning sterility, drug-device combination approvals, and quality assurance, present a significant challenge for manufacturers
- Developing and manufacturing prefilled syringes involves complex processes, specialized equipment, and compliance with evolving international standards, which significantly increase production costs
- These factors create entry barriers for new players and can delay product launches, particularly for smaller pharmaceutical companies aiming to enter the market
For instance,
- According to a 2024 report by PharmTech, companies developing combination products such as prefilled syringes must navigate dual regulatory pathways involving both drug and device compliance under FDA and EMA frameworks, increasing the time and cost associated with market approval
- Consequently, high costs associated with production, quality control, and regulatory compliance can restrict innovation, limit affordability, and reduce access—particularly in cost-sensitive and emerging markets—ultimately restraining market growth
Prefilled Syringes Market Scope
The market is segmented on the basis of product type, material type, closing system, design, application, and end user
|
Segmentation |
Sub-Segmentation |
|
By Product Type |
|
|
By Material Type |
|
|
By Closing system |
|
|
By Design |
|
|
By Application |
|
|
By End User |
|
In 2025, glass prefilled syringes is projected to dominate the market with a largest share in material type segment
The glass prefilled syringes segment is expected to dominate the prefilled syringes market with the largest share of 50.9% in 2025 due to its superior chemical resistance, excellent barrier properties, and compatibility with a wide range of drug formulations, including biologics. Glass syringes also offer enhanced drug stability, reducing the risk of interaction between the container and the medication. Their long-standing reliability in high-precision drug delivery further contributes to their widespread adoption.
The vaccines and immunizations is expected to account for the largest share during the forecast period in application market
In 2025, vaccines and immunizations segment is expected to dominate the market with the largest market share of 26.4% due to the rising global demand for efficient vaccine delivery, especially for mass immunization programs. Prefilled syringes enhance safety, reduce dosing errors, and ensure faster administration, which is critical during large-scale public health campaigns. In addition, their convenience and reduced risk of contamination make them ideal for both routine and emergency vaccination effort.
Prefilled Syringes Market Regional Analysis
“North America Holds the Largest Share in the Prefilled Syringes Market”
- North America dominates the prefilled syringes market with a market share of estimated 32.5%, driven, by advanced healthcare infrastructure, widespread adoption of injectable biologics, and the high prevalence of chronic diseases such as diabetes and rheumatoid arthritis
- U.S. holds a market share of 27.7%, due to increasing demand for self-administered treatments, the presence of leading pharmaceutical companies, and a large aging population that requires ongoing medication
- The availability of favorable reimbursement policies and continuous advancements in drug delivery technologies further strengthen the market in North America
- In addition, the growing preference for home healthcare services and the rise of personalized medicine are fueling market growth across the region
“Asia-Pacific is Projected to Register the Highest CAGR in the Prefilled Syringes Market”
- Asia-Pacific is expected to witness the highest growth rate in the prefilled syringes market with a market share of 25.3%, driven by rapid healthcare infrastructure development, increased focus on chronic disease management, and rising healthcare awareness
- Countries such as China, India, and Japan are emerging as key markets due to their large populations and the increasing prevalence of chronic conditions such as diabetes, cancer, and cardiovascular diseases
- China, with its growing healthcare investments and expanding diabetic patient base, is expected to be the largest contributor to market growth in the region
- India is projected to register the highest CAGR of 13.9%, driven by improved access to healthcare, rising disposable income, and increasing adoption of modern drug delivery systems such as prefilled syringes
Prefilled Syringes Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- BD(U.S.)
- Gerresheimer AG(Germany)
- Schott AG(Germany)
- YPSOMED(Switzerland)
- Sandoz Group AG(Switzerland)
- Medtronic (Ireland)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Hikma Pharmaceuticals PLC (U.K.)
- Pfizer Inc. (U.S.)
- AstraZeneca (U.K.)
- Bausch Health Companies Inc. (Canada)
- Amgen Inc. (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- West Pharmaceutical Services, Inc. (U.S.)
- Biocon (India)
- Sanofi (France)
- Johnson & Johnson Services, Inc. (U.S.)
- Viatris Inc. (U.S.)
- B. Braun SE (Germany)
- Fresenius Kabi AG (Germany)
Latest Developments in Global Prefilled Syringes Market
- In April 2025, The U.S. Food and Drug Administration (FDA) approved a prefilled syringe version of Argenx SE's immune disorder drug, Vyvgart. This approval enables at-home self-administration for patients with generalized myasthenia gravis (gMG) and chronic inflammatory demyelinating polyneuropathy (CIDP). The new formulation, branded as Vyvgart Hytrulo, utilizes efgartigimod with Halozyme Therapeutics’ delivery system and is expected to be available within two weeks at a comparable cost to the existing subcutaneous version
- In December 2024, Genentech announced that the FDA had approved the Vabysmo (faricimab-svoa) 6.0 mg single-dose prefilled syringe for treating wet age-related macular degeneration (AMD), diabetic macular edema (DME), and macular edema following retinal vein occlusion (RVO). This approval marks the first and only bispecific antibody approved for the eye, offering rapid and robust vision improvements
- In July 2024, BD (Becton, Dickinson, and Company) unveiled a next-generation glass refillable syringe designed to meet the stringent requirements of vaccine performance, including processability, cosmetics, contamination, and integrity. This innovation aims to enhance vaccine delivery and manufacturing processes
- In September 2024, Simtra BioPharma Solutions announced a $250+ million investment to expand its sterile fill/finish manufacturing facilities in Bloomington, Indiana. The new facility will house high-speed automated isolator syringe fill lines and a dedicated clinical line to support the growing demand for prefilled syringes
- In December 2020, BD (Becton, Dickinson and Company), a leading global medical technology company, announced plans to invest USD 1.2 billion over four years. This investment is made to expand and upgrade manufacturing capacity and technology for pre-fillable syringes (PFS) and advanced drug delivery systems (ADDS) at its six global manufacturing locations and open a new manufacturing facility in Europe. By the end of 2023, the new manufacturing site in Europe should be operating
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.