Global Trop 2 Targeted Drug Conjugate Market
시장 규모 (USD 10억)
연평균 성장률 :
% 
 USD
3.36 Billion
USD
11.09 Billion
2025
2033
USD
3.36 Billion
USD
11.09 Billion
2025
2033
| 2026 –2033 | |
| USD 3.36 Billion | |
| USD 11.09 Billion | |
|
|
|
|
Global Trop-2 Targeted Drug Conjugate Market Segmentation, By Drug Type (Antibody-Drug Conjugates, Monoclonal Antibodies, and Bispecific Antibodies), Application (Breast Cancer, Non-Small Cell Lung Cancer, Urothelial Carcinoma, and Other Cancers), End User (Academic & Research Hospitals, Oncology Specialty Clinics, Contract Research Organizations, and Diagnostics Labs)- Industry Trends and Forecast to 2033
Trop-2 Targeted Drug Conjugate Market Size
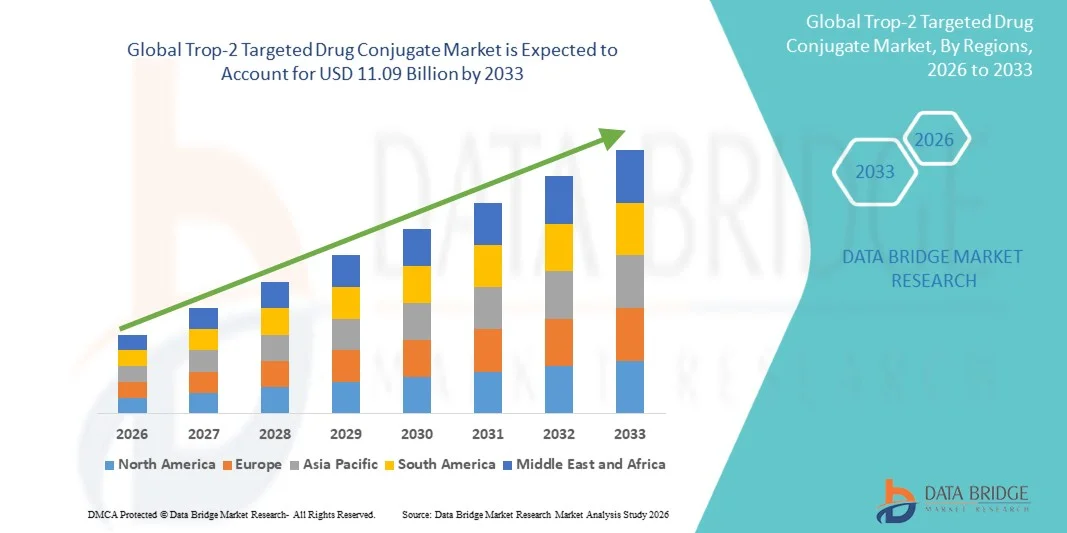
- The global Trop-2 targeted drug conjugate market size was valued at USD 3.36 billion in 2025 and is expected to reach USD 11.09 billion by 2033, at a CAGR of 16.10% during the forecast period
- The market growth is largely driven by the increasing prevalence of Trop-2–expressing solid tumors and rapid advances in antibody-drug conjugate (ADC) technologies, enabling more precise and effective targeted cancer therapies
- Furthermore, rising demand for treatments with improved efficacy and reduced systemic toxicity, along with strong clinical trial activity and regulatory approvals in oncology, is positioning Trop-2 targeted drug conjugates as a key therapeutic option, thereby significantly accelerating overall market growth
Trop-2 Targeted Drug Conjugate Market Analysis
- Trop-2 targeted drug conjugates, which combine Trop-2–specific antibodies with potent cytotoxic payloads, are becoming increasingly vital in modern oncology treatment due to their targeted delivery mechanism, improved clinical efficacy, and reduced off-target toxicity compared to conventional chemotherapy across multiple solid tumor indications
- The escalating demand for Trop-2 targeted drug conjugates is primarily fueled by the rising global burden of cancers such as breast, lung, and urothelial cancer, along with rapid advancements in antibody-drug conjugate (ADC) technology and expanding clinical validation across earlier lines of therapy
- North America dominated the Trop-2 targeted drug conjugate market with the largest revenue share of 47% in 2025, characterized by early regulatory approvals, strong biopharmaceutical R&D capabilities, and a robust oncology care ecosystem, with the U.S. witnessing significant uptake driven by high clinical trial activity and rapid commercialization of novel ADC therapies
- Asia-Pacific is expected to be the fastest growing region in the Trop-2 targeted drug conjugate market during the forecast period due to increasing cancer incidence, improving access to advanced oncology treatments, expanding clinical research infrastructure, and rising investments from domestic and multinational biopharma companies
- The antibody-drug conjugate (ADC) segment dominated the Trop-2 targeted drug conjugate market with a market share of 60.9% in 2025, driven by strong clinical outcomes, regulatory approvals, and a deep development pipeline targeting Trop-2–overexpressing tumors
Report Scope and Trop-2 Targeted Drug Conjugate Market Segmentation
|
Attributes |
Trop-2 Targeted Drug Conjugate Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Trop-2 Targeted Drug Conjugate Market Trends
“Expansion of Trop-2 ADCs Across Multiple Solid Tumor Indications”
- A significant and accelerating trend in the global Trop-2 targeted drug conjugate market is the rapid expansion of antibody-drug conjugates (ADCs) into multiple solid tumor indications beyond initial late-line settings, driven by strong clinical efficacy and differentiated targeting mechanisms
- For instance, Gilead Sciences’ Trodelvy (sacituzumab govitecan) has expanded from triple-negative breast cancer into HR+/HER2- breast cancer and urothelial cancer, demonstrating the broad therapeutic potential of Trop-2 targeting
- Advances in ADC design, including optimized linker technologies and more potent yet tolerable payloads, are enabling improved safety profiles and enhanced tumor selectivity, allowing developers to evaluate Trop-2 ADCs in earlier lines of therapy
- The growing number of combination studies pairing Trop-2 ADCs with immunotherapies or targeted agents is further strengthening their clinical positioning, supporting improved response rates and longer progression-free survival
- This trend toward broader indication coverage and combinational use is reshaping clinical development strategies in oncology, positioning Trop-2 ADCs as backbone therapies across several epithelial cancers
- Consequently, biopharmaceutical companies are increasingly prioritizing Trop-2 ADC pipeline expansion, with multiple candidates advancing through mid- to late-stage clinical trials across global markets
- The increasing use of biomarker-driven patient selection to identify high Trop-2 expression tumors is further enhancing clinical success rates and market adoption
Trop-2 Targeted Drug Conjugate Market Dynamics
Driver
“Rising Cancer Burden and Strong Clinical Validation of ADC Therapies”
- The increasing global prevalence of solid tumors such as breast, lung, and urothelial cancers, combined with unmet needs in treatment-resistant patient populations, is a key driver accelerating demand for Trop-2 targeted drug conjugates
- For instance, positive Phase II and Phase III clinical outcomes demonstrating survival benefits and manageable toxicity profiles have supported regulatory approvals and rapid clinical adoption of Trop-2 ADCs
- As oncologists seek therapies that offer higher precision and reduced systemic toxicity compared to conventional chemotherapy, Trop-2 ADCs are gaining strong acceptance across specialized cancer centers
- Furthermore, growing investment by pharmaceutical companies in oncology R&D and ADC platform technologies is accelerating pipeline development and commercialization timelines
- The increasing inclusion of Trop-2 targeted therapies in treatment guidelines and clinical pathways is further reinforcing market growth across major oncology markets
- Expanding reimbursement coverage for high-value oncology biologics in developed markets is also supporting wider patient access and uptake
- In parallel, favorable regulatory pathways such as accelerated approvals for breakthrough oncology drugs are shortening time-to-market for Trop-2 ADC candidates
Restraint/Challenge
“High Development Costs and Safety-Related Clinical Challenges”
- The complex manufacturing processes and high development costs associated with antibody-drug conjugates present a significant challenge to widespread market expansion, particularly for smaller biotechnology firms
- For instance, ensuring consistent conjugation quality, payload stability, and scalable production remains technically demanding and capital intensive
- Safety concerns such as off-target toxicity, neutropenia, and gastrointestinal adverse effects observed in some Trop-2 ADC trials can limit dosing flexibility and require careful patient management
- In addition, stringent regulatory requirements for biologics and ADCs can prolong approval timelines, increasing development risk and overall commercialization costs
- Overcoming these challenges through improved ADC engineering, better patient selection strategies, and continued investment in manufacturing innovation will be critical for sustained growth of the Trop-2 targeted drug conjugate market
- Limited long-term real-world safety data for newer Trop-2 ADCs may slow physician confidence in broader patient populations
- Pricing pressure from payers due to the high cost of ADC therapies can also restrict adoption, particularly in cost-sensitive healthcare systems
Trop-2 Targeted Drug Conjugate Market Scope
The market is segmented on the basis of drug type, application, and end user.
- By Drug Type
On the basis of drug type, the Trop-2 targeted drug conjugate market is segmented into antibody-drug conjugates, monoclonal antibodies, and bispecific antibodies. The antibody-drug conjugates (ADCs) segment dominated the market in 2025 with a market share of 60.9%, driven by their superior clinical efficacy achieved through targeted delivery of potent cytotoxic agents directly to Trop-2–expressing cancer cells. ADCs offer a favorable balance between efficacy and safety, reducing systemic toxicity compared to conventional chemotherapy. The commercial success and regulatory approvals of leading Trop-2 ADCs have strengthened physician confidence and accelerated adoption across oncology centers. In addition, a strong late-stage clinical pipeline and expanding indications in breast and urothelial cancers continue to reinforce ADC dominance. Robust investment by major biopharmaceutical companies in ADC platforms further supports this segment’s leadership.
The bispecific antibodies segment is expected to witness the fastest growth during the forecast period, owing to their ability to engage multiple biological targets simultaneously and enhance immune-mediated tumor cell killing. These therapies offer novel mechanisms of action that can potentially overcome resistance seen with single-target agents. Growing R&D focus on next-generation immuno-oncology approaches is accelerating early-stage development of Trop-2 bispecifics. Increasing interest from biotech firms and academic institutions is driving innovation in this space. As clinical data matures, bispecific antibodies are expected to gain traction as complementary or combination therapies.
- By Application
On the basis of application, the market is segmented into breast cancer, non-small cell lung cancer, urothelial carcinoma, and other cancers. The breast cancer segment dominated the market in 2025, primarily due to the high prevalence of Trop-2 expression in breast tumors and the early clinical and commercial success of Trop-2 ADCs in this indication. Triple-negative and HR+/HER2- breast cancer patients have shown meaningful survival benefits from Trop-2–targeted therapies. Established treatment guidelines and strong oncologist familiarity further support adoption. Ongoing trials evaluating use in earlier treatment lines continue to expand patient eligibility. This segment benefits from strong reimbursement support in major healthcare markets.
The non-small cell lung cancer (NSCLC) segment is anticipated to grow at the fastest rate over the forecast period, driven by the rising global incidence of lung cancer and increasing identification of Trop-2 expression in NSCLC patients. Limited treatment options for advanced-stage and treatment-resistant NSCLC create a strong unmet clinical need. Expanding clinical trial activity evaluating Trop-2 ADCs in combination with immunotherapies is accelerating development. Improved diagnostic testing for biomarker identification is further supporting patient selection. These factors collectively position NSCLC as a high-growth application area.
- By End User
On the basis of end user, the market is segmented into academic & research hospitals, oncology specialty clinics, contract research organizations, and diagnostics labs. The academic & research hospitals segment dominated the market in 2025, supported by their central role in conducting clinical trials, early adoption of innovative oncology therapies, and access to specialized infrastructure. These institutions are often the first to administer newly approved Trop-2 ADCs and generate real-world clinical evidence. Strong collaboration with pharmaceutical companies enables rapid translation of research into clinical practice. Availability of multidisciplinary oncology expertise further drives patient referrals. This segment also benefits from government and institutional research funding.
The oncology specialty clinics segment is expected to register the fastest growth during the forecast period, driven by the decentralization of cancer care and increasing outpatient administration of targeted biologics. These clinics offer specialized, patient-centric treatment settings with shorter wait times and focused oncology expertise. Growing adoption of ADCs in routine clinical practice supports volume growth in these facilities. Expansion of specialty clinics in emerging markets is further boosting demand. Improved reimbursement coverage for advanced oncology therapies is also accelerating uptake in this segment.
Trop-2 Targeted Drug Conjugate Market Regional Analysis
- North America dominated the Trop-2 targeted drug conjugate market with the largest revenue share of 47% in 2025, characterized by early regulatory approvals, strong biopharmaceutical R&D capabilities, and a robust oncology care ecosystem
- Healthcare providers in the region highly value the clinical efficacy, precision targeting, and improved safety profiles offered by Trop-2 drug conjugates compared to conventional chemotherapy options
- This widespread adoption is further supported by advanced oncology infrastructure, significant biopharmaceutical R&D investment, favorable reimbursement frameworks, and a strong clinical trial ecosystem, positioning Trop-2 targeted drug conjugates as preferred treatments across major cancer centers
U.S. Trop-2 Targeted Drug Conjugate Market Insight
The U.S. Trop-2 targeted drug conjugate market captured the largest revenue share within North America in 2025, driven by high cancer incidence, early regulatory approvals, and rapid adoption of advanced antibody-drug conjugate (ADC) therapies. Oncology centers increasingly prioritize targeted treatments that offer improved efficacy and manageable safety profiles for difficult-to-treat cancers. The strong presence of leading biopharmaceutical companies, along with extensive clinical trial activity, continues to accelerate innovation and commercialization. Moreover, favorable reimbursement policies and strong physician awareness are significantly contributing to sustained market expansion.
Europe Trop-2 Targeted Drug Conjugate Market Insight
The Europe Trop-2 targeted drug conjugate market is projected to expand at a substantial CAGR during the forecast period, primarily driven by rising cancer prevalence and increasing adoption of precision oncology therapies. Growing emphasis on personalized medicine and strong regulatory support for innovative biologics are fostering market growth. European healthcare systems are increasingly incorporating ADCs into oncology treatment pathways. The region is witnessing notable uptake across academic hospitals and specialized cancer centers, supported by collaborative research initiatives and expanding access to novel therapies.
U.K. Trop-2 Targeted Drug Conjugate Market Insight
The U.K. Trop-2 targeted drug conjugate market is anticipated to grow at a noteworthy CAGR over the forecast period, driven by advancements in oncology research and strong government support for innovative cancer treatments. Increasing focus on targeted and biologic therapies within the National Health Service is supporting adoption. In addition, rising awareness of novel ADC therapies among oncologists is encouraging clinical uptake. The U.K.’s strong clinical trial ecosystem is expected to continue stimulating market growth.
Germany Trop-2 Targeted Drug Conjugate Market Insight
The Germany Trop-2 targeted drug conjugate market is expected to expand at a considerable CAGR during the forecast period, fueled by advanced healthcare infrastructure and high investment in cancer research. Germany’s strong emphasis on innovation and precision medicine supports the adoption of targeted biologics such as ADCs. The presence of leading academic research centers and pharmaceutical companies further strengthens market development. Increasing use of biomarker-driven therapies aligns well with the country’s focus on evidence-based oncology care.
Asia-Pacific Trop-2 Targeted Drug Conjugate Market Insight
The Asia-Pacific Trop-2 targeted drug conjugate market is poised to grow at the fastest CAGR during the forecast period, driven by rising cancer incidence, expanding healthcare access, and increasing investment in oncology R&D across countries such as China, Japan, and India. Growing clinical trial activity and regulatory reforms aimed at accelerating drug approvals are boosting market momentum. The region’s emergence as a biopharmaceutical manufacturing and research hub is improving affordability and availability of advanced therapies. These factors collectively support rapid market expansion across Asia-Pacific.
Japan Trop-2 Targeted Drug Conjugate Market Insight
The Japan Trop-2 targeted drug conjugate market is gaining momentum due to the country’s advanced healthcare system, strong focus on innovation, and aging population with rising cancer burden. Japanese clinicians place high value on targeted therapies that improve treatment outcomes while minimizing toxicity. The integration of ADCs into oncology practice is supported by active clinical research and early adoption of novel biologics. In addition, strong collaboration between academia and industry continues to drive market growth.
India Trop-2 Targeted Drug Conjugate Market Insight
The India Trop-2 targeted drug conjugate market accounted for a significant share within Asia-Pacific in 2025, supported by increasing cancer prevalence, expanding oncology infrastructure, and growing access to advanced biologic therapies. India’s rapidly developing pharmaceutical and biotech sector is actively participating in clinical research and manufacturing of complex biologics. Rising awareness of targeted cancer treatments among healthcare professionals is driving adoption. Government initiatives to strengthen cancer care and improve access to innovative therapies are further propelling market growth in India.
Trop-2 Targeted Drug Conjugate Market Share
The Trop-2 Targeted Drug Conjugate industry is primarily led by well-established companies, including:
- Gilead Sciences, Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- Alphamab Oncology (China)
- Daiichi Sankyo (Japan)
- Astellas Pharma Inc. (Japan)
- Seagen Inc. (U.S.)
- Pfizer Inc. (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Amgen Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- AstraZeneca (U.K.)
- Novartis AG (Switzerland)
- Eli Lilly and Company (U.S.)
- Sanofi (France)
- Takeda Pharmaceutical Company Limited (Japan)
- Zai Lab Limited (China)
- Crescent Biopharma, Inc. (U.S.)
- MedImmune, LLC (U.S.)
- Janssen Biotech, Inc. (U.S.)
- Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (China)
What are the Recent Developments in Global Trop-2 Targeted Drug Conjugate Market?
- In June 2025, AstraZeneca and Daiichi Sankyo announced the approval of Datroway for locally advanced or metastatic EGFR-mutated non-small cell lung cancer (NSCLC) in the U.S., broadening the clinical utility of Trop-2 targeted ADCs into lung cancer after prior EGFR-directed therapy and chemotherapy
- In March 2025, industry analysis highlighted that three Trop-2 targeted therapies Trodelvy, sacituzumab tirumotecan, and Datroway have been approved and set the stage for a new generation of Trop-2 targeted cancer treatments, with more than 40 additional therapies in clinical trials, underscoring rapidly growing research and commercial interest in this therapeutic class
- In January 2025, the FDA approved Datroway (datopotamab deruxtecan), a Trop-2-directed antibody-drug conjugate from Daiichi Sankyo and AstraZeneca, for adults with unresectable or metastatic hormone receptor-positive (HR+), HER2-negative breast cancer, marking a key commercial milestone for Trop-2 ADCs
- In November 2024, Sichuan Kelun-Biotech’s Trop-2 ADC sacituzumab tirumotecan (sac-TMT) received first-ever marketing authorization in China for adult patients with unresectable locally advanced or metastatic triple-negative breast cancer (TNBC) following positive phase III OptiTROP-Breast01 results, representing the first domestically developed Trop-2 ADC approved in China
- In February 2023, the U.S. Food and Drug Administration (FDA) approved sacituzumab govitecan-hziy (Trodelvy) for adults with unresectable locally advanced or metastatic HR-positive, HER2-negative breast cancer who have received endocrine-based therapy and at least two additional systemic therapies, significantly expanding the indication for this Trop-2-targeted ADC beyond triple-negative breast cancer
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.