Global Advanced Therapy Medicinal Products Market
Размер рынка в млрд долларов США
CAGR :
% 
 USD
34.65 Billion
USD
93.43 Billion
2024
2032
USD
34.65 Billion
USD
93.43 Billion
2024
2032
| 2025 –2032 | |
| USD 34.65 Billion | |
| USD 93.43 Billion | |
|
|
|
|
Global Advanced Therapy Medicinal Products Market Segmentation, By Therapy Type (CAR-T Therapy, Cell Therapy, Tissue Engineered Product, Gene Therapy, and Others), Product Type (Tissue Engineered Products, Somatic Cell Treatment Product, Joined ATMPs, and Others), Applications (Muscular Dystrophies, Alzheimer’s, Hemophilia, Cystic Fibrosis, and Others), End-Users (Clinic, Hospital, and Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, and Online Pharmacy) – Industry Trends and Forecast to 2032
Advanced Therapy Medicinal Products Market Analysis
In the advanced therapy medicinal products (ATMPs) market, the latest methods and technological advancements are shaping the future of medical treatments. One of the key innovations is gene editing technologies, such as CRISPR-Cas9, which allows for precise genetic modifications to treat genetic disorders. Cell therapy and tissue engineering are also advancing, with stem cell-based treatments showing promise for regenerating damaged tissues and organs. In addition, viral vectors and nanoparticles are being explored for safer and more effective gene delivery systems.
These technologies are driving growth by offering solutions for previously untreatable conditions, such as inherited genetic disorders, certain cancers, and neurological diseases. For instance, gene therapies for conditions such as sickle cell anemia and muscular dystrophy are seeing increased approvals from regulatory bodies such as the FDA and EMA.
The market is expected to experience significant growth, with advancements in manufacturing processes making therapies more cost-effective and scalable. The global market for ATMPs is forecasted to expand due to rising investments, regulatory support, and increasing clinical trial successes, presenting substantial opportunities for developers.
Advanced Therapy Medicinal Products Market Size
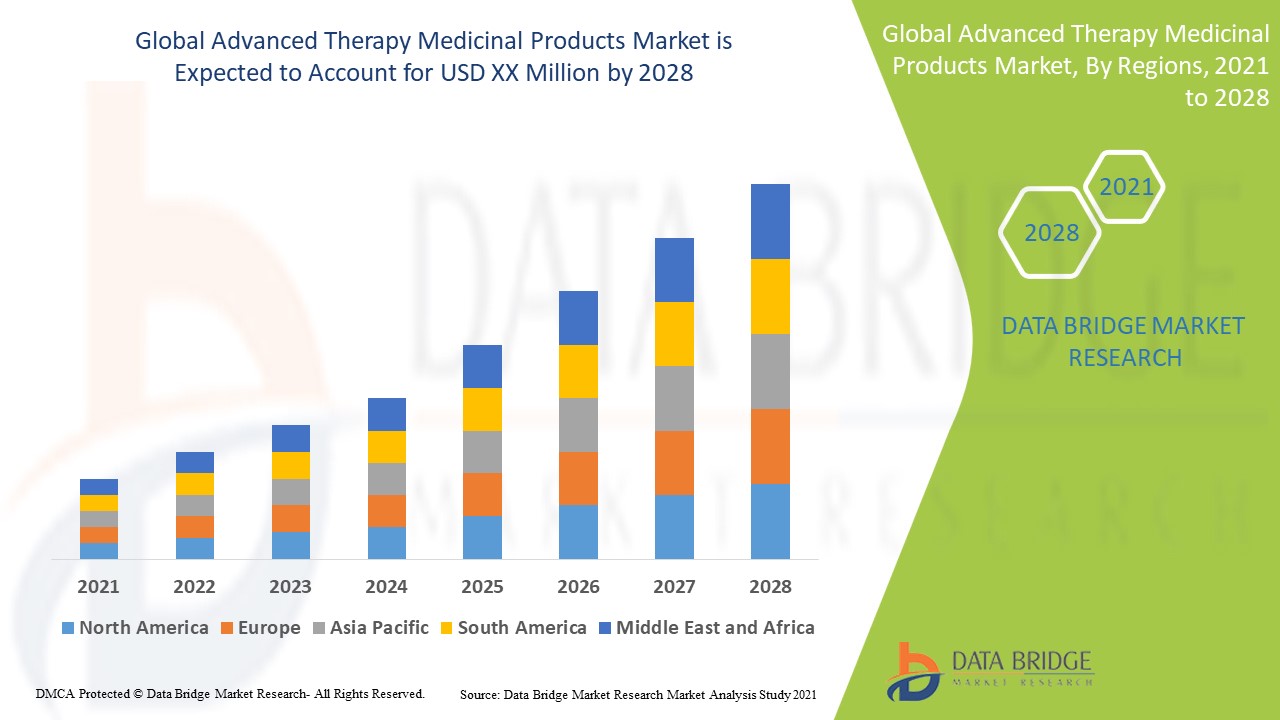
The global advanced therapy medicinal products market size was valued at USD 34.65 billion in 2024 and is projected to reach USD 93.43 billion by 2032, with a CAGR of 13.20% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Advanced Therapy Medicinal Products Market Trends
“Growth of Gene Therapies”
One specific trend driving the growth of the Advanced Therapy Medicinal Products (ATMP) market is the increasing focus on gene therapies. These therapies aim to treat genetic disorders by modifying or replacing faulty genes, Zolgensma, a gene therapy for spinal muscular atrophy (SMA) treatment, which has shown significant success in treating this rare and life-threatening condition. The growing pipeline of gene therapies, backed by advancements in CRISPR and viral vector technology, is fueling market expansion. Regulatory bodies such as the FDA are also providing accelerated approvals, further boosting market growth. This trend is expected to continue as novel therapies emerge for genetic conditions and rare diseases.
Report Scope and Advanced Therapy Medicinal Products Market Segmentation
|
Attributes |
Advanced Therapy Medicinal Products Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Key Market Players |
uniQure N.V. (Netherlands), Pfizer Inc. (U.S.), bluebird bio, Inc. (U.S.), BioMarin (U.S.), Novartis AG (Switzerland), General Electric Company (U.S.), Takeda Pharmaceutical Company Limited (Japan), Gilead Sciences, Inc. (U.S.), Spark Therapeutics, Inc. (U.S.), Kolon TissueGene, Inc. (South Korea), JCR Pharmaceuticals Co., Ltd. (Japan), Medipost (South Korea), Vericel Corporation (U.S.), Pharmicell Co., Ltd. (South Korea), Organogenesis Inc. (U.S.), Abeona Therapeutics Inc. (U.S.), Humacyte, Inc. (U.S.), and Bristol-Myers Squibb Company (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Advanced Therapy Medicinal Products Market Definition
Advanced Therapy Medicinal Products (ATMPs) are a group of innovative treatments that include gene therapies, cell therapies, and tissue-engineered products. These therapies aim to treat or prevent diseases by modifying or repairing cells or tissues at a molecular or cellular level. ATMPs can offer cures for conditions that have limited or no existing treatment options, such as genetic disorders, certain cancers, and degenerative diseases. They often require specialized manufacturing techniques and rigorous clinical trials. With the potential to revolutionize healthcare, ATMPs are closely regulated to ensure safety, efficacy, and quality in their use for patients.
Advanced Therapy Medicinal Products Market Dynamics
Drivers
- Increasing Prevalence of Chronic Diseases
The increasing prevalence of chronic diseases is a significant driver for the Advanced Therapy Medicinal Products (ATMPs) market. Chronic conditions such as cancer, genetic disorders, and cardiovascular diseases are rising globally, creating an urgent need for innovative therapies. ATMPs, including gene therapies and cell-based treatments, provide potential cures and improved outcomes for these conditions. For instance, Kymriah, a CAR-T cell therapy developed for certain types of leukemia, demonstrates how ATMPs can offer breakthrough treatment where traditional therapies fall short. As the burden of chronic diseases grows, the demand for such advanced therapies increases, pushing the market forward and offering hope for long-term treatment solutions for previously untreatable conditions.
- Increasing Personalized Medicine Demand
The growing demand for personalized medicine is a significant driver of the Advanced Therapy Medicinal Products (ATMPs) market. Personalized treatments, tailored to an individual's genetic makeup, offer more effective solutions than traditional therapies. ATMPs, such as gene therapies and cell therapies, are at the forefront of this shift, providing precise and targeted treatments. For instance, CAR-T cell therapies such as Kymriah, approved by the FDA, have demonstrated success in treating specific types of leukemia and lymphoma by using a patient's own immune cells. This personalized approach not only enhances efficacy but also minimizes side effects, propelling the demand for ATMPs as a preferred option in modern healthcare.
Opportunities
- Improved Manufacturing Processes
Advances in manufacturing technologies have significantly reduced the costs and complexity of producing Advanced Therapy Medicinal Products (ATMPs), creating opportunities for market growth. For instance, the development of scalable gene-editing platforms and automation in cell therapy production has improved efficiency and lowered production costs. Companies such as Autolus Therapeutics are adopting innovative manufacturing techniques that enhance the speed and cost-effectiveness of gene and cell therapies. As manufacturing becomes more efficient, it enables wider access to these therapies, reducing the cost per patient and expanding their availability to underserved populations. This creates a broader market opportunity for companies to scale up production and reach new patient demographics.
- Rising Investment in Biotechnology
Increased funding and venture capital in biotechnology are creating significant opportunities for the Advanced Therapy Medicinal Products (ATMPs) market. This influx of investment is enabling biotech companies to accelerate the development of novel gene and cell therapies. For instance, companies such as Bluebird Bio and Spark Therapeutics have benefited from substantial financial backing to advance their gene therapies for genetic disorders. Such investments are driving innovation, improving research capabilities, and facilitating the commercialization of ATMPs. This surge in capital is expected to foster breakthroughs, expand treatment options for rare diseases, and enhance manufacturing processes, thereby increasing the accessibility and affordability of ATMPs globally.
Restraints/Challenges
- High Development Costs
High development costs are a major restraint for the advanced therapy medicinal products (ATMPs) market. The production of therapies such as gene and cell therapies involves complex and highly specialized processes, such as cell culturing and genetic engineering, which require significant financial investment. In addition, the long and rigorous regulatory approval pathways contribute to rising costs. This combination of expensive development and manufacturing processes leads to high therapy prices, making it difficult for these products to become widely accessible. The burden of these costs not only limits the affordability for healthcare systems but also hinders the adoption of ATMPs, preventing their widespread use and limiting market growth.
- Complexities in Manufacturing
The manufacturing of advanced therapy medicinal products (ATMPs) presents significant challenges due to the complexity of production processes. These therapies require specialized facilities for cell cultures, gene editing, and personalized treatments, all of which are susceptible to variability and quality control issues. The intricate nature of these processes makes scaling up production difficult, leading to inefficiencies and higher costs. Moreover, the specialized equipment and skilled labor needed add to the expense. These manufacturing complexities not only hinder cost-effectiveness but also disrupt the stability of the supply chain, creating delays and limiting the availability of products. Consequently, these challenges pose a major restraint to the growth of the ATMP market.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Advanced Therapy Medicinal Products Market Scope
The market is segmented on the basis of therapy type, product type, application, end-users and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Therapy Type
- CAR-T Therapy
- Cell Therapy
- Tissue Engineered Product
- Gene Therapy
- Others
Product Type
- Tissue Engineered Products
- Somatic Cell Treatment Product
- Joined ATMPs
- Others
Applications
- Muscular Dystrophies
- Alzheimer’s
- Hemophilia
- Cystic Fibrosis
- Others
End-Users
- Clinic
- Hospital
- Others
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
Advanced Therapy Medicinal Products Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, therapy type, product type, application, end-users and distribution channel as referenced above.
The countries covered in the market report are U.S., Canada, Mexico in North America, Germany, Sweden, Poland, Denmark, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe in Europe, Japan, China, India, South Korea, New Zealand, Vietnam, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in Asia-Pacific (APAC), Brazil, Argentina, Rest of South America as a part of South America, U.A.E, Saudi Arabia, Oman, Qatar, Kuwait, South Africa, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA)
North America is expected to dominate the advanced therapy medicinal products market due to its presence of major key players, high research and development spending, favorable regulations, successful drugs, substantial healthcare expenditure, and advancements in gene and cell therapy within the region.
Asia-Pacific is expected to show growth in the advanced therapy medicinal products market due to increasing research and development activities, rising investments in the healthcare sector, and a growing number of skin burn cases and cancer diseases, driving demand for advanced treatments.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Advanced Therapy Medicinal Products Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Advanced Therapy Medicinal Products Market Leaders Operating in the Market Are:
- uniQure N.V. (Netherlands)
- Pfizer Inc. (U.S.)
- bluebird bio, Inc. (U.S.)
- BioMarin (U.S.)
- Novartis AG (Switzerland)
- General Electric Company (U.S.)
- Takeda Pharmaceutical Company Limited (Japan)
- Gilead Sciences, Inc. (U.S.)
- Spark Therapeutics, Inc. (U.S.)
- Kolon TissueGene, Inc. (South Korea)
- JCR Pharmaceuticals Co., Ltd. (Japan)
- Medipost (South Korea)
- Vericel Corporation (U.S.)
- Pharmicell Co., Ltd. (South Korea)
- Organogenesis Inc. (U.S.)
- Abeona Therapeutics Inc. (U.S.)
- Humacyte, Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
Latest Developments in Advanced Therapy Medicinal Products Market
- In August 2023, Orchard Therapeutics announced positive long-term results from an updated integrated analysis of 39 patients with metachromatic leukodystrophy (MLD) treated with the investigational gene therapy, OTL-200. This promising data supports the potential of gene therapy as an effective treatment option, instilling greater confidence in gene therapies. The success will accelerate further investments and innovation within the sector, advancing the development of novel therapies for rare diseases
- In April 2022, The FDA expanded its guidance for the development, manufacture, and control of advanced biological products, including chimeric antigen receptor (CAR) T cell therapies. This update provides clearer recommendations to developers and manufacturers, outlining key steps in CAR-T product development. The move aims to streamline the regulatory process and foster the growth of cutting-edge therapies, including personalized treatments for cancers and other complex conditions
- In February 2021, Bristol Myers Squibb’s Breyanzi, a CAR-T cell therapy, received FDA approval for treating adults with relapsed or refractory large B-cell lymphoma. The approval marks a significant milestone for CAR-T therapies, offering patients with difficult-to-treat cancers a new, potentially life-saving treatment option. The therapy uses genetically modified T cells to target and destroy cancer cells, representing a breakthrough in cancer immunotherapy
- In January 2021, Organogenesis Holding Inc. received FDA Regenerative Medicine Advanced Therapy (RMAT) designation for ReNu, a cryopreserved amniotic suspension allograft designed to treat knee osteoarthritis symptoms. The RMAT designation expedites the development of regenerative medicine treatments, facilitating faster clinical trials and regulatory approval. ReNu’s potential to alleviate knee pain and improve joint function positions it as a promising treatment for patients suffering from osteoarthritis
- In January 2021, FUJIFILM Diosynth Biotechnologies announced a $40 million investment in a new process research and manufacturing facility for innovative medicines and viral vectors. This investment underscores the growing demand for advanced manufacturing capabilities in the biotechnology industry, particularly for the production of viral vectors used in gene and cell therapies. The new facility will help address the increasing need for scalable production solutions for cutting-edge therapeutics
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.