Cancer is a serious illness that results from unchecked, exponential cell proliferation that disturbs and damages organs. This illness has been acknowledged as one of the major problems facing health care and medicine. The most common cancer therapies are chemotherapy, surgery, and radiotherapy. Different cancer treatment methods have a variety of adverse effects. Conventional therapy approaches have been shown to have a number of negative side effects, low specificity and sensitivity, small therapeutic windows, and, more recently, the formation of tumor cells resistant to such treatments. Bacteria are a class of prokaryotic microorganisms that offer enormous promise for use in the treatment of cancer. At the moment, scientists are interested in using microbes to cure cancer.
Bacteria are promising candidates and live micro-medication for cancer therapies due to their high potential for genetic modification to make them non-pathogenic, their unique virulence factors (which can be used as weapons against tumors), their capacity to proliferate in tissues, and the potential to control their population by administering antibiotics. However, the major obstacles to using bacteria-based approaches for cancer treatment are their potential cytotoxicity effects, inability to lyse malignant cells completely, and the possibility of genome alterations. Currently, researchers are looking into and creating brand-new cancer treatment plans. To choose the finest alternative therapies and use them in cancer treatment, however, careful analysis and changes to these approaches are needed.
As a result, innovative therapeutic modalities such as immunotherapy, stem cell-based therapy, hormone therapy, and immunotherapy based on dendritic cells have been put into practice lately. Chemotherapy, a tried-and-true method of treating malignancies, can be useful in conjunction with other multimodal therapies. However, in some patients, it may also lead to the development of multidrug-resistant (MDR) malignant cells and metastases. Therefore, there is a huge need for modern cancer treatment strategies and therapeutics that are more effective while having less side effects.
The cancer immunotherapy market is expected to witness market growth at a rate of 13.5% in the forecast period of 2022 to 2029. Data Bridge Market Research report on cancer immunotherapy market provides analysis and insights regarding the various factors expected to be prevalent throughout the forecast period while providing their impacts on the market's growth. The rise in the prevalence of heart disorder globally is escalating the growth of cancer immunotherapy market.
To know more about the study, visit: https://www.databridgemarketresearch.com/reports/global-cancer-immunotherapy-market
The Concept
It has been known for a century that bacteria can treat cancer. Live, attenuated, or genetically altered obligatory or facultative anaerobic bacterial species have the innate ability to colonize tumors and are able to reproduce in the tumors with specificity, preventing the formation of malignant cells. For hundreds of years, spontaneous tumor regression has been linked to microbial infection, which has sparked interest in using bacteria as an anti-cancer treatment. Bone sarcoma surgeon Dr. William B. Coley (1862–1936) was a pioneer in the use of "Coley's toxins," a combination of heat-killed bacteria and live bacteria, to treat his patients. Coley's labor was unfortunately stopped for almost 50 years when he was compelled to cease. Several bacterial species are being produced at the moment to fight cancer.
Key elements in defining a bacterial species' anti-tumor activity in vivo are their genetic makeup, infectious behavior, and tumor microenvironment. Since the late 1970s, the only bacterial treatment for superficial, non-muscle invasive bladder cancer (NMIBC) that has received FDA approval is Bacillus Calmette-Guerin (BCG). Mycobacterium bovis was attenuated and acquired as the BCG strain at the Pasteur Institute in the early 1900s. Usually, live bacteria are repeatedly injected into the bladder of the patient. Although the response prediction factors of BCG are unknown, it is advised as the gold standard of therapy for high-risk NMIBC and continues to be the most successful intravesical treatment for this condition.
Numerous traits that are displayed by bacteria could be useful in the treatment of cancer. The biochemical interactions between the bacteria and the human tumor microenvironment are what give rise to the direct and immune-mediated anti-cancer effects. Important characteristics of the bacteria, including their motility, tumor chemotaxis, invasiveness, cytotoxic capability, and pathogen-associated molecular patterns (PAMP) composition/abundance, differ between strains and may have an impact on how they cause the anti-tumor response.
Numerous cancer types now have a better chance of being cured because of recent advancements in cancer therapies like targeted therapy and immunotherapy. Toxicities to normal tissue and cells, issues in treating deep tumor tissue, and the potential for drug resistance in tumor cells are ongoing obstacles to developing novel treatment techniques. Live tumor-targeting bacteria utilization offers a novel therapeutic choice that overcomes these difficulties. Tumor-targeting microorganisms are more adaptable at suppressing cancer than the majority of treatments.
Bacteria preferentially gather and multiply inside tumors, where they can trigger anti-cancer immune responses. Bacteria can be further trained to generate and distribute anti-cancer medicines based on clinical requirements using straightforward genetic engineering or advanced synthetic bioengineering. To improve clinical outcomes, therapeutic strategies utilizing live tumor-targeting bacteria can be used either alone or in combination with existing anti-cancer treatments. In animal tumor models, live tumor-targeting bacteria can specifically colonize tumors or tumor-driven lymph nodes, suppress tumor growth, and increase survival time following systemic infection. For instance, the most well-known attenuated strain of Salmonella Typhimurium, VNP20009, has a tumor: liver colonization ratio greater than 1000:1 and exhibits strong inhibitory effects on tumor growth and metastasis in mouse models. It has been attenuated more than 10,000 times compared to the wild-type strain. Tumor-targeting bacteria can maximize the effects of chemotherapy medications while minimizing systemic harm to the patient and overcoming penetration barriers. Cytokines, cytotoxic drugs, immunomodulators, prodrug-converting enzymes, and short-interfering RNAs are examples of potential payloads for targeted cancer delivery (siRNAs). It is conceivable further to restrict the buildup of anti-cancer payloads at tumor locations and to manage the timing of drug delivery by controlling bacterial gene expression.
Mechanisms that Microorganisms Use to Hunt Down and Inhibit Cancers
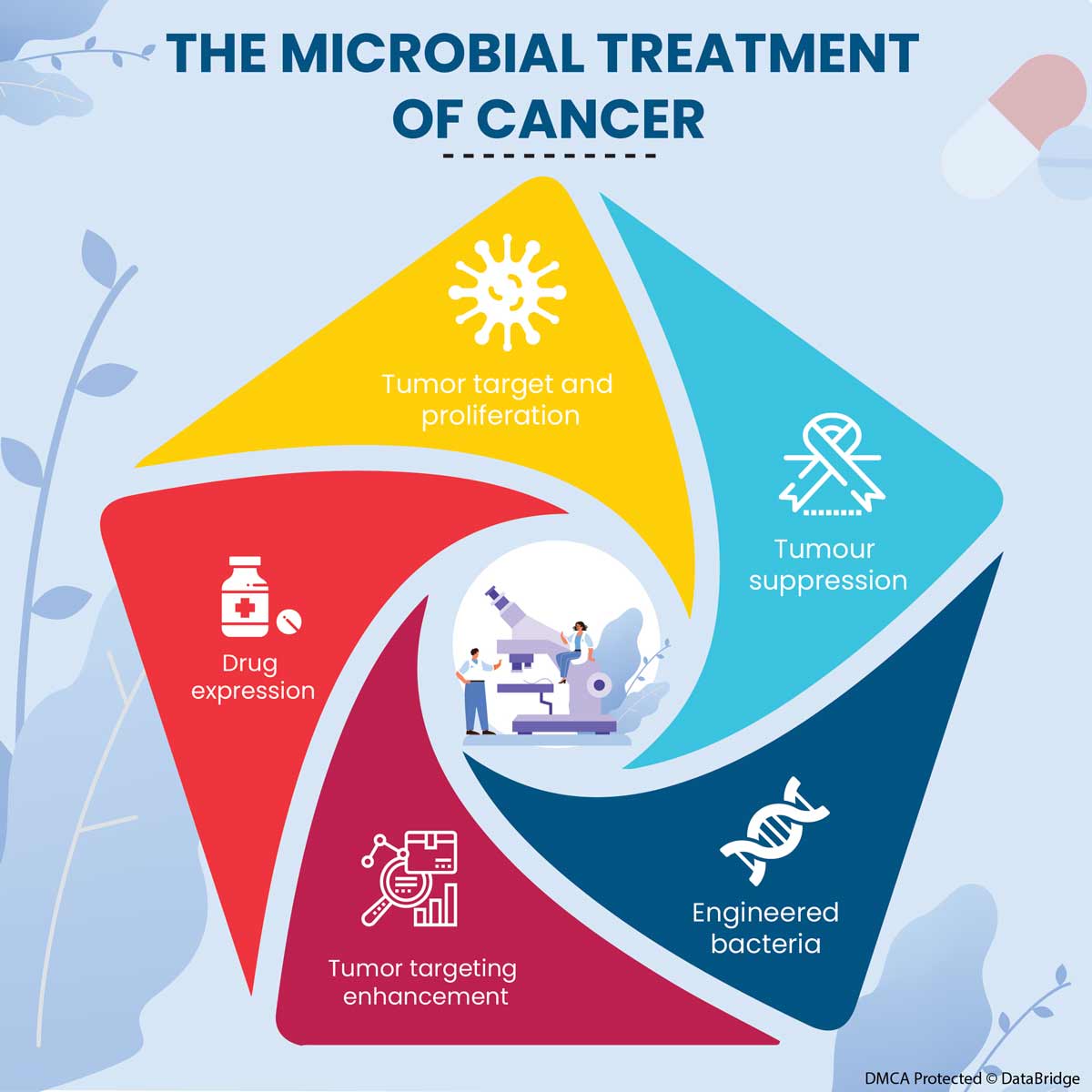
Fig.1: Mechanisms Used by Microorganisms to Target Tumors
- Tumor Target and Proliferation
The primary benefit of bacteria-based cancer therapy is its capacity to target tumors with particularity through specialized mechanisms. At the moment, it is believed that bacteria use both passive and active pathways to enter tumor tissue from blood circulation. Bacteria may first passively entrap themselves in the disordered tumor vasculature before flowing into the tumor due to inflammation brought on by a sudden rise in tumor necrosis factor (TNF) levels in the tumor arteries. In reality, the active and passive processes can both be used by bacteria to precisely target tumors; they are neither strain dependent nor mutually exclusive. The host immune system plays a key role in Listeria spp tumor-targeting strategy.
Listeria cells directly infect myeloid-derived suppressor cells (MDSCs), which can subsequently transport bacteria to TMEs, as well as antigen-presenting cells like dendritic cells (DCs) or macrophages. Listeria cells in MDSCs are shielded from immune clearance by this particular mechanism, whereas Listeria cells in healthy tissue milieus are quickly cleared. Motility is a crucial characteristic that permits germs to enter tumor tissue more deeply. Bacteria are sophisticated living organisms that can obtain energy from their surrounding environment, in contrast to the passive dispersion and restricted penetration inherent to chemotherapeutic medications. As a result, their transport capacity is entropically boundless. Live bacteria can multiply vigorously after successfully targeting and penetrating tumors. A tumor: normal organ bacterial ratio of more than 10,000:120 was seen in a study using tumor-bearing animals. Typhimurium cells reached greater than 1 1010 CFU/g of tumor tissue 3 days after intravenous administration. These bacteria were still countable after 10 days.
- Tumour Suppression
A number of different methods induces tumor regression caused by bacterial overgrowth. In TMEs, several bacterial strains exhibit various tumor suppression strategies. Salmonella spp. produce toxins or starve tumor cells of nutrients to cause apoptosis and/or autophagy, resulting in the immediate death of tumor cells. Additionally, Salmonella infection can cause the universal protein Connexin 43 (Cx43) to be upregulated in tumor cells, encouraging the development of gap junctions between tumor cells and dendritic cells (DCs). Listeria spp. can directly destroy tumor cells due to their inherent pathogenic abilities, which include activating nicotinamide adenine dinucleotide phosphate oxidase and raising intracellular calcium levels, which result in large quantities of reactive oxygen species (ROS).
Listeria spp. have been shown to have a dual mechanism of action; they can directly infiltrate tumor cells or indirectly damage tumors by suppressing MDSCs' immune systems. An immune-stimulating phenotype is simultaneously induced in a subpopulation of Listeria-carrying MDSCs via increased IL-12 production, which then supports enhanced T and NK cell responses, which then target Listeria-infected tumor cells. Both investigations demonstrated that CD8+ T lymphocytes could effectively eliminate tumor cells in both primary and metastatic cancers. In conclusion, it is hypothesized that bacterial infection contributes most significantly to tumor regression by activating a complex immune cell population in TMEs and its intrinsic anti-cancer effects. It is obvious that bacteria likely offer a distinct immunotherapy technique that can be amplified by sophisticated genetic engineering of bacterial strains, despite the fact that the basic mechanism varies.
- Engineered Bacteria
It is also crucial to engineering bacteria to reduce their pathogenicity toward the host immune system. Certain virulence factors may cause the inherent anti-cancer activity of some bacteria; it should be highlighted. In order to maintain their anti-cancer action, attenuation must be performed. For instance, significant virulence genes have been deleted in human infections to transform fatally deadly strains into harmless variants. The msbB and purI genes were deleted to create the attenuated S. Typhimurium strain VNP20009, which has been extensively researched in tumor-bearing mice and exhibits potential tumor-targeting specificity and tumor-suppressive properties. The incidence of septic shock is significantly decreased by the deletion of msbB in the Salmonella genus. This deletion also significantly reduces the induction of TNF by LPS.
A further strategy for enhancing tumor-specific proliferation with virulence attenuation involves the introduction of certain nutrient-dependent mutations in bacteria. The auxotrophic for leucine and arginine A1-R Salmonella strain preferentially colonizes tumors has anti-cancer properties and makes tumors more susceptible to chemotherapy. The dal/dat locus was inactivated to create a L. monocytogenes strain that is auxotrophic for the amino acid D-alanine found in cell walls. This mutant strain could activate cytotoxic T lymphocytes and was severely attenuated.
- Tumor Targeting Enhancement
The engineering techniques used to enhance bacterial tumor targeting can improve anti-cancer efficacy and safety. The ppGpp-deficient strain SHJ2037 was genetically altered to express tumor-specific ligands on the cell surface to achieve these results. For the purpose of promoting the production of outer membrane protein A on the bacterial surface, an Arg-Gly-Asp peptide that binds to v3 integrin was fused to it. In MDA-MB-231 breast cancer cells and MDA-MB-435 melanoma xenografts overexpressing v3 integrin, the resultant strain displayed improved tumor selectivity and noticeably higher anti-cancer efficacy. Additionally, tumor-associated antigens such the lymphoma-associated antigen CD20 and carcinoembryonic antigen have been targeted by bacteria. These strains lowered non-specific bacterial buildup in the liver and spleen and demonstrated potent anti-cancer effects.
By boosting the bacteria's injection capacity without reducing their intrinsic qualities, probiotic strains demonstrated better tumor selectivity. Symbioflor-2 probiotic E. coli cells were promptly eliminated from the liver and spleen, and they only persisted in the tumor, demonstrating effective tumor targeting. Mice infected with a probiotic Salmonella strain tolerated a high bacterial load without displaying any pathological symptoms, but because of the strain's poor therapeutic efficacy—despite its excellent safety in vivo—improvements in the payload delivery system are required.
- Drug Expression
Since the majority of the payloads supplied by bacteria that target tumors are hazardous to both normal and malignant cells, precise control over their production is preferred to constitutive expression. Payload expression can be precisely triggered to optimize therapeutic effects while reducing systemic toxicity. Theoretically, by introducing a certain promoter sequence upstream of a gene encoding a medication, one can confer transcriptional control via external inputs and create a programmable gene expression system. A system like this enables control over the place and time of drug synthesis in vivo. The methods used to trigger this sort of gene often fall into one of the following three categories: internal, self (quorum sensing-QS), or external.
TMEs differ from normal tissue in that they have unique characteristics including necrosis, acidity, and hypoxia that bacteria may detect and take advantage of to increase tumor specificity. For instance, fumarate and nitrate decrease in the hypoxic environment within tumor tissue triggers the activation of hypoxia-inducible promoters such those of HIP-1 and pepT. The tumor-to-normal tissue ratio in TMEs might surpass 10,000, which makes it possible to use QS as a switch for the expression of certain genes. An auto inducer, the artificial LuxI protein, and the transcriptional regulatory protein LuxR control one practical QS system. AHL, a product of LuxI that depends on bacterial density, activates LuxR and encourages transcription of the genes it targets. Highly expressed heterologous proteins have been produced in bacteria-colonizing tumors using AHL concentration-dependent QS systems.
Conclusion
The two-edged sword of cancer treatment is bacteria. It is possible to use bacteria as a cancer treatment, and solid tumors may respond well to this approach. However, this medication's negative, irreversible side effects prevented its widespread clinical usage. Some attenuated kinds of bacteria that can treat cancer have recently been identified and explored in an effort to combat these negative effects. These bacterial species are thought to have negligible to no negative effects when used in cancer therapy. While the therapeutic potential of bacteria alone may not be fully realized, their alterations as anti-tumor agents, anti-oncogenes, or immunogenic antigens, as well as their combination with other therapeutic processes, will increase their potential for cancer therapy. Further research is required to examine the clinical importance of bacteria-based cancer therapy because the field of employing bacteria as an anti-cancer agent is still relatively young. According to the review's conclusions, this cancer therapy must be improved and explored further.
Data Bridge Market Research analyses that the cancer tumor profiling market is expected to undergo a CAGR of 12.75% during the forecast period. This indicates that the market value, which was USD 9.35 billion in 2021, would rocket up to USD 24.44 billion by 2029. "Immunoassays" dominates the technology segment of the cancer tumor profiling market owing to rising application in conduct tumor profiling on a large scale as they help measure the presence and concentration of analytes in a sample.
To know more about the study, visit: https://www.databridgemarketresearch.com/reports/global-cancer-tumor-profiling-market









