Mercado europeo de dispositivos de neuroestimulación interna, por tipo de producto ( estimulación de la médula espinal (SCS), estimulación cerebral profunda , estimulación del nervio vago, estimulación del nervio sacro y estimulación eléctrica gástrica ), canal de distribución (licitación directa y proveedor de servicios de terceros): tendencias de la industria y pronóstico hasta 2029.
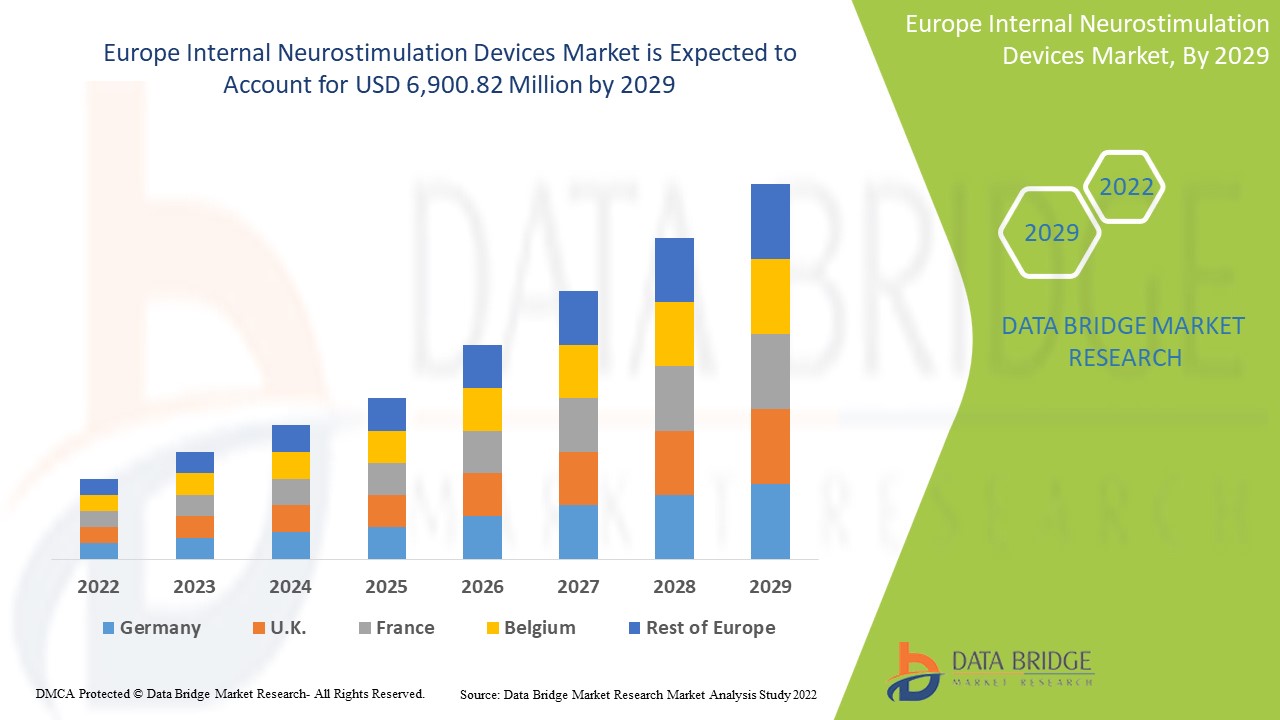
Análisis y perspectivas del mercado de dispositivos de neuroestimulación interna en Europa
Se espera que el aumento de la demanda de dispositivos de neuroestimulación interna como terapia complementaria, el aumento de la prevalencia e incidencia de enfermedades neurológicas, el aumento de la financiación para los dispositivos de neuroestimulación, los avances tecnológicos en los dispositivos de neuroestimulación interna y el aumento de las aprobaciones de productos impulsen el crecimiento del mercado.


Se espera que las iniciativas estratégicas de los actores del mercado y el aumento de la financiación de los actores públicos y privados para los dispositivos de neuroestimulación interna creen oportunidades para el crecimiento del mercado. Sin embargo, se espera que la falta de expertos capacitados y calificados en dispositivos de neuroestimulación interna limite el crecimiento del segmento. Se espera que la disponibilidad de dispositivos de diagnóstico por imagen alternativos desafíe el crecimiento del mercado.
Data Bridge Market Research analiza que el mercado europeo de dispositivos de neuroestimulación interna crecerá a una CAGR del 18,0% y USD 6.900,82 millones durante el período de pronóstico de 2022 a 2029.
|
Métrica del informe |
Detalles |
|
Período de pronóstico |
2022 a 2029 |
|
Año base |
2021 |
|
Años históricos |
2020 (Personalizable para 2019 - 2024) |
|
Unidades cuantitativas |
Ingresos en millones de USD, precios en USD |
|
Segmentos cubiertos |
Por tipo de producto ( estimulación de la médula espinal (SCS), estimulación cerebral profunda , estimulación del nervio vago, estimulación del nervio sacro y estimulación eléctrica gástrica ), canal de distribución (licitación directa y proveedor de servicios externo) |
|
Países cubiertos |
Alemania, Reino Unido, Italia, España, Francia, Suiza, Bélgica, Países Bajos, Rusia, Turquía y el resto de Europa |
|
Actores del mercado cubiertos |
Medtronic, LivaNova PLC, Abbott, Microsemi (una subsidiaria de Microchip Technology Inc.), Boston Scientific Corporation, Inspire Medical Systems, Inc., Integer Holdings Corporation, Finetech Medical, ALEVA NEUROTHERAPEUTICS, OptoGenTech GmbH., INBRAIN NEUROELECTRONICS, Neuronano AB, NEURIMPULSE srl, Newronika SpA, NEVRO CORP., Nalu Medical, Inc., Valencia Technologies, CIRTEC, Sequana Medical NV, BIOINDUCTION, ONWARD, Axonics, Inc., Mainstay Medical, entre otros. |
Definición de mercado
Un dispositivo de neuroestimulación interna es un dispositivo que se coloca quirúrgicamente. Envía señales eléctricas suaves al espacio epidural cerca de la columna vertebral a través de uno o más cables delgados llamados derivaciones. La neuroestimulación alivia el dolor al interrumpir las señales de dolor que viajan entre la médula espinal y el cerebro.
Los dispositivos de neuroestimulación incluyen métodos invasivos y no invasivos que implican estimulación eléctrica para impulsar la función neuronal dentro de un circuito. La mayor demanda de dispositivos de neuroestimulación interna se debe a los avances tecnológicos de última generación en los dispositivos de neuroestimulación, que brindan un alivio terapéutico muy necesario a una cantidad sin precedentes de personas afectadas por trastornos neurológicos y psiquiátricos debilitantes en todo el mundo. El auge de las terapias de neuromodulación modernas ha aumentado a lo largo de medio siglo, rico en descubrimientos fortuitos y avances tecnológicos que han llevado a diferentes estrategias de neuroestimulación. En las últimas dos décadas, la innovación en la tecnología de dispositivos médicos ha comenzado a impulsar la evolución de estos sistemas de neuroestimulación a un ritmo más acelerado.
El dispositivo de neuroestimulación interna que utilizan los pacientes es la estimulación del nervio vago. El dispositivo de neuroestimulación vago utiliza un dispositivo para estimular el nervio vago con impulsos eléctricos. Actualmente, la FDA ha aprobado un estimulador del nervio vago implantable para tratar la epilepsia y la depresión. Hay un nervio vago en cada lado del cuerpo, que va desde el tronco encefálico a través del cuello hasta el pecho y el abdomen. En el futuro, los avances en software como la estimulación de circuito cerrado y la programación remota permitirán que los dispositivos de neuroestimulación interna sean una tecnología más personalizada y accesible. Se espera que el futuro de los dispositivos de neuroestimulación interna mejore aún más la calidad de vida.
Dinámica del mercado de dispositivos de neuroestimulación interna en Europa
En esta sección se aborda la comprensión de los factores impulsores, las oportunidades, las limitaciones y los desafíos del mercado. Todo esto se analiza en detalle a continuación:
Conductores
- Aumento de la demanda de implantes de dispositivos de neuroestimulación interna en las clínicas de neurología de Europa
La menor demanda de recursos sanitarios por parte de los pacientes que reciben neuroestimulación sugiere que el tratamiento de estimulación de los nervios periféricos y de la médula espinal, aunque se asocia a unos costes iniciales relativamente elevados, demuestra importantes beneficios económicos a largo plazo en Europa. La neuroestimulación debería considerarse una opción viable para el tratamiento precoz de pacientes con dolor neuropático crónico intratable.
Por ejemplo,
- El Centro Médico Universitario de Friburgo en Alemania proporciona implantes neurológicos a los pacientes
La mayor demanda de dispositivos de neuroestimulación interna implantables en neurología significa un aumento de las inversiones relacionadas con la investigación y el desarrollo para el descubrimiento y desarrollo de dispositivos de neuroestimulación interna avanzados y sin dolor, lo que se espera que impulse el crecimiento del mercado.
- Aumento de la financiación para los dispositivos de neuroestimulación
El objetivo de la financiación de los dispositivos de neuroestimulación interna es fomentar las solicitudes destinadas a desarrollar la próxima generación de dispositivos de estimulación cerebral para el tratamiento de trastornos de salud mental. Los nuevos dispositivos deben ir más allá de la estimulación eléctrica o magnética existente y desarrollar nuevas técnicas de estimulación capaces de aumentar la precisión espaciotemporal y los enfoques multifocales de circuito cerrado.
Por ejemplo,
- En febrero de 2022, Nalu Medical recaudó alrededor de 104 millones de dólares en financiación para la tecnología de neuroestimulación. La financiación se realizó para avanzar en sus soluciones mínimamente invasivas para pacientes con dolor neuropático crónico.
La financiación por parte del gobierno se traduciría en seguridad para el paciente y ahorro de costes. Además, los hospitales y las agencias de atención sanitaria administrarían este tratamiento a un precio más bajo gracias a la colaboración con las organizaciones gubernamentales. Por tanto, se espera que los avances en las actividades de investigación y desarrollo y la financiación por parte del gobierno impulsen el crecimiento del mercado.
Oportunidades
- Iniciativas estratégicas de los actores del mercado
La demanda de dispositivos de neuroestimulación interna está aumentando en el mercado debido a la investigación y el desarrollo y al crecimiento del mercado de dispositivos de neuroestimulación interna, impulsado por el deseo de medicamentos innovadores. Por lo tanto, los principales actores del mercado han implementado una nueva estrategia mediante el desarrollo de nuevos dispositivos y equipos, la colaboración con otros actores del mercado y la mejora de las operaciones comerciales y la rentabilidad.
Por ejemplo,
- En enero de 2021, Boston Scientific comenzó a enviar sus sistemas de activación de la línea espinal Wave Writer Alpha a los EE. UU.
- En mayo de 2019, Abbott Laboratories se asoció con el NIH en la iniciativa BRAIN (Investigación del cerebro a través del avance de neurotecnologías innovadoras) para acelerar los avances en la ciencia neurológica.
Por lo tanto, las empresas que operan a nivel mundial en el mercado de dispositivos de neuroestimulación están adoptando la colaboración para aumentar su cartera de productos con productos ricos en tecnología avanzada para impulsar su negocio en varias dimensiones. Por lo tanto, se espera que las iniciativas estratégicas de los actores clave del mercado ofrezcan oportunidades significativas para los actores del mercado que operan en el mercado europeo de dispositivos de neuroestimulación interna.
- Avances tecnológicos en los dispositivos de neuroestimulación interna
Los avances tecnológicos en dispositivos de neuroestimulación interna utilizan tecnología de neuromodulación, que aplica directamente agentes eléctricos o farmacéuticos a una zona específica. Los dispositivos y tratamientos de neuromodulación y neuroestimulación interna cambian la vida. Los avances tecnológicos modulan las funciones neuronales de manera programable y regulan la actividad neuronal desordenada. Los resultados se pueden obtener con un error mínimo.
- En mayo de 2022, ONWARD presentó el generador de pulsos implantable (IPG) ARC para estimular la médula espinal y restaurar el movimiento y la función autónoma de las personas con LME y otras afecciones que afectan la movilidad.
El aumento de las aplicaciones tecnológicas en los dispositivos de resonancia magnética cerebral se traducirá en menos recursos humanos y un diagnóstico y una recuperación más rápidos de las enfermedades neurológicas crónicas. En el futuro, la tecnología de inteligencia artificial sustituirá a las máquinas de resonancia magnética manuales abiertas y cerradas. Este aspecto se convertirá en una fuerza impulsora del mercado europeo de dispositivos de neuroestimulación.
Restricciones/Desafíos
- Riesgos asociados a la implantación de estos dispositivos
Los implantes médicos conllevan varios riesgos, incluidos los relacionados con la cirugía durante la instalación o extracción, la infección y el fallo del implante. Los materiales utilizados en los implantes pueden provocar reacciones en algunas personas. Todo procedimiento quirúrgico conlleva algún riesgo, como hematomas, molestias, hinchazón y enrojecimiento en el lugar de la cirugía. Por lo tanto, obstaculizará proporcionalmente el crecimiento de los dispositivos de implantes. Por lo tanto, la creciente conciencia sobre los crecientes riesgos relacionados con los implantes de neuroestimulación puede obstaculizar el crecimiento del mercado.
Por ejemplo,
- Fallo del implante
- Riesgos quirúrgicos durante la colocación o extracción
- Riesgo de secuestro de dispositivos implantables
- Aumento de infecciones
- Los materiales utilizados en los implantes podrían presentar reacciones adversas en los pacientes
Los riesgos mencionados anteriormente pueden obstaculizar el crecimiento del mercado europeo de dispositivos de neuroestimulación interna, ya que los riesgos que implican para los pacientes son de suma importancia. Por lo tanto, la conciencia de los riesgos potenciales de los dispositivos implantables representa un desafío para el mercado europeo de dispositivos de neuroestimulación interna.
- Falta de profesionales sanitarios cualificados
Los dispositivos de neuroestimulación son dispositivos médicos implantables y programables que aplican estimulación eléctrica a partes específicas del cerebro, la médula espinal o el sistema nervioso periférico del paciente para ayudar a tratar diversas afecciones, como el dolor crónico, los trastornos del movimiento, la epilepsia y la enfermedad de Parkinson. La aplicación de estas potentes tecnologías requiere un procedimiento de desarrollo de alto costo y profesionales capacitados para manipular dispositivos sensibles. El reemplazo debe realizarse cada 3 a 6 años después de la implantación, lo que supone una carga económica y, por lo general, de bolsillo para la mayoría de los pacientes.
Sólo un número relativamente pequeño de pacientes en los países más pobres puede permitirse la terapia neurológica debido a los altos precios, un entorno de reembolso débil y una falta de recursos sanitarios cualificados. Como resultado, los centros sanitarios dudan en gastar dinero en tecnología novedosa o de vanguardia, lo que limita la expansión del mercado interno de neuroestimulación. Por lo tanto, estos desafíos obstaculizan el crecimiento del mercado.
Impacto de la COVID-19 en el mercado europeo de dispositivos de neuroestimulación interna
Durante la pandemia, el mercado europeo de dispositivos de neuroestimulación interna se centra en el uso de una combinación de biología y tecnología de la información. Durante la pandemia de COVID-19, se han añadido nuevos desafíos terapéuticos a los habituales en los dispositivos de neuroestimulación interna. Los pacientes con un dispositivo implantable para infusión intratecal necesitan una recarga de la bomba para evitar el síndrome de abstinencia. Los pacientes con implantes de neuroestimulación pueden necesitar controles en caso de infección, dehiscencia de la herida o migración del cable.
Acontecimientos recientes
- En enero de 2022, Medtronic recibió la aprobación de la Administración de Alimentos y Medicamentos (FDA) para el neuroestimulador recargable Intellis, una terapia de estimulación de la médula espinal para tratar el dolor crónico resultante de la neuropatía periférica diabética. La aprobación del producto recibida resultó en la incorporación de un nuevo producto en la categoría de productos de estimulación de la médula espinal. Se espera que la aprobación se apruebe después de su comercialización en el mercado estadounidense.
- En julio de 2022, Abbott recibió la aprobación de la Administración de Alimentos y Medicamentos (FDA) para el sistema de estimulación cerebral profunda Infinity para tratar la depresión. La aprobación recibida dio como resultado el aumento de la iniciación de aprobaciones previas y posteriores a la comercialización y la incorporación de un nuevo producto a la cartera de productos.
Alcance del mercado de dispositivos de neuroestimulación interna en Europa
El mercado europeo de dispositivos de neuroestimulación interna se clasifica por tipo de producto y canal de distribución. El crecimiento entre estos segmentos le ayudará a analizar segmentos de crecimiento reducido en las industrias y brindará a los usuarios una valiosa descripción general del mercado y conocimientos del mercado para ayudarlos a tomar decisiones estratégicas para identificar las principales aplicaciones del mercado.
Tipo de producto
- Estimulación de la médula espinal (SCS)
- Estimulación cerebral profunda
- Estimulación del nervio vago
- Estimulación del nervio sacro
- Estimulación eléctrica gástrica
Sobre la base del tipo de producto, el mercado europeo de dispositivos de neuroestimulación interna está segmentado en estimulación de la médula espinal (SCS), estimulación cerebral profunda, estimulación del nervio vago , estimulación del nervio sacro y estimulación eléctrica gástrica.
Canal de distribución
- Licitación directa
- Proveedor de servicios de terceros
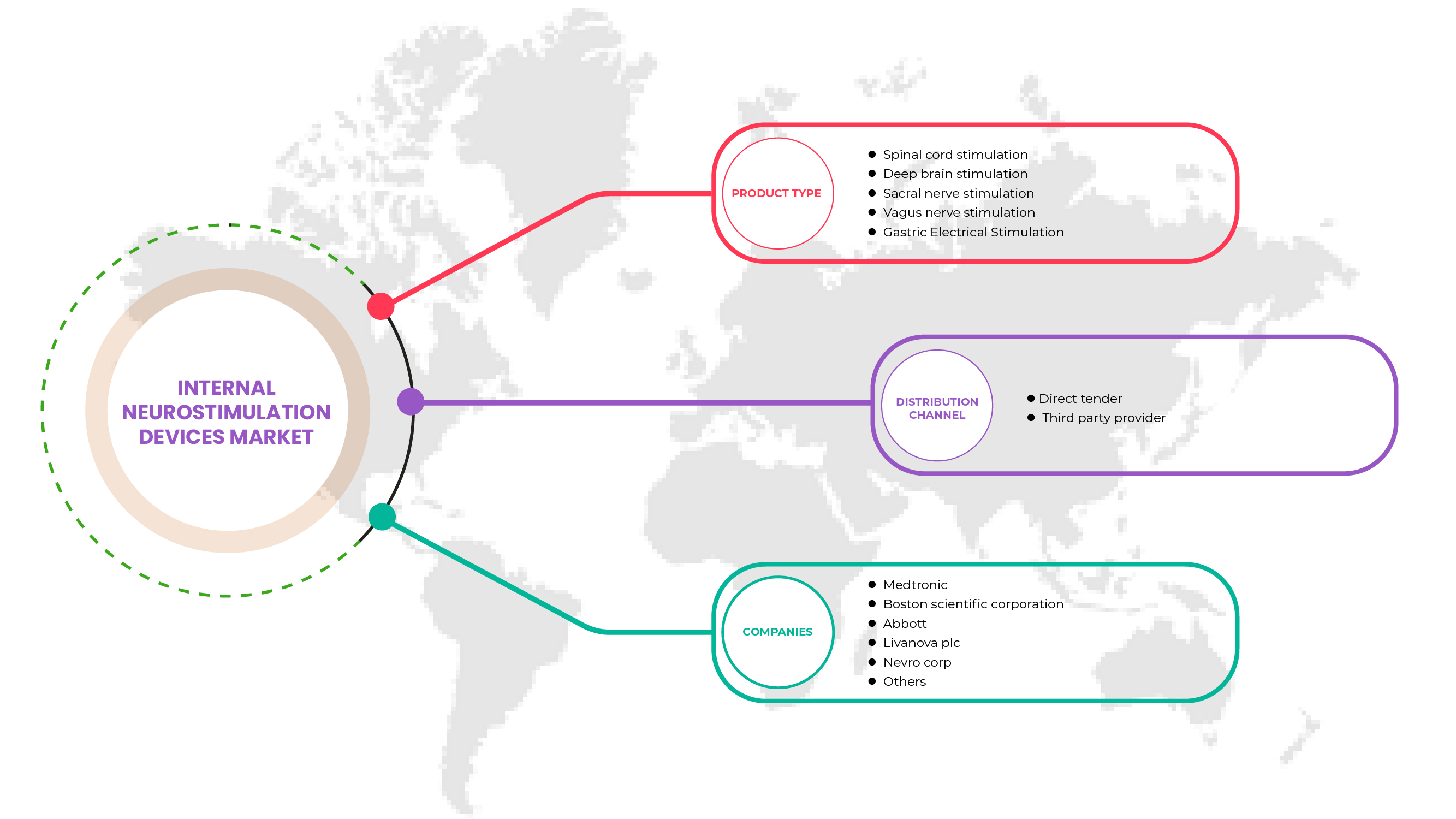
Sobre la base del canal de distribución, el mercado interno de dispositivos de neuroestimulación en Europa está segmentado en licitación directa y proveedor de servicios de terceros.
Análisis y perspectivas regionales del mercado de dispositivos de neuroestimulación interna en Europa
Se analiza el mercado europeo de dispositivos de neuroestimulación interna y las regiones proporcionan información sobre el tamaño del mercado y las tendencias por país, tipo de producto y canal de distribución, como se menciona anteriormente.
Algunos de los países incluidos en el mercado europeo de dispositivos de neuroestimulación interna son Alemania, Reino Unido, Italia, España, Francia, Suiza, Bélgica, Países Bajos, Rusia, Turquía y el resto de Europa. Se espera que Alemania domine el mercado debido al aumento de la actividad de investigación y desarrollo, los requisitos de dispositivos de neuroestimulación interna integrados y la creciente demanda de dispositivos de neuroestimulación avanzados. Iniciativas estratégicas de los actores del mercado en Alemania en el desarrollo de dispositivos de neuroestimulación interna y una mayor demanda de dispositivos de neuroestimulación de la médula espinal.
La sección de países del informe también proporciona factores individuales que impactan en el mercado y cambios en las regulaciones en el mercado a nivel nacional que afectan las tendencias actuales y futuras del mercado. Los puntos de datos como nuevas ventas, ventas de reemplazo, demografía del país, epidemiología de enfermedades y aranceles de importación y exportación son algunos de los principales indicadores utilizados para pronosticar el escenario del mercado para países individuales. Además, la presencia y disponibilidad de marcas europeas y sus desafíos enfrentados debido a la gran o escasa competencia de las marcas locales y nacionales que impactan los canales de venta se consideran al proporcionar un análisis de pronóstico de los datos del país.
Análisis del panorama competitivo y de la cuota de mercado de los dispositivos de neuroestimulación interna en Europa
El panorama competitivo del mercado europeo de dispositivos de neuroestimulación interna proporciona detalles sobre los competidores. Los detalles incluyen una descripción general de la empresa, sus finanzas, los ingresos generados, el potencial de mercado, la inversión en investigación y desarrollo, las nuevas iniciativas de mercado, la presencia en Europa, los sitios e instalaciones de producción, las capacidades de producción, las fortalezas y debilidades de la empresa, el lanzamiento de productos, la amplitud y variedad de productos y el dominio de las aplicaciones. Los puntos de datos anteriores proporcionados solo están relacionados con el enfoque de las empresas en relación con el mercado europeo de dispositivos de neuroestimulación interna.
Algunos de los principales actores que operan en el mercado europeo de dispositivos de neuroestimulación interna son Medtronic, LivaNova PLC, Abbott, Microsemi (una subsidiaria de Microchip Technology Inc.), Boston Scientific Corporation, Inspire Medical Systems, Inc., Integer Holdings Corporation, Finetech Medical, ALEVA NEUROTHERAPEUTICS, OptoGenTech GmbH., INBRAIN NEUROELECTRONICS, Neuronano AB, NEURIMPULSE srl, Newronika SpA, NEVRO CORP., Nalu Medical, Inc., Valencia Technologies, CIRTEC, Sequana Medical NV, BIOINDUCTION, ONWARD, Axonics, Inc., Mainstay Medical, entre otros.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHIC SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 RESEARCH METHODOLOGY
2.6 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.7 DBMR MARKET POSITION GRID
2.8 THE CATEGORY VS TIME GRID
2.9 SECONDARY SOURCES
2.1 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL
4.2 PORTER'S FIVE FORCES MODEL
4.3 PIPELINE ANALYSIS FOR INTERNAL NEUROSTIMULATION DEVICES MARKET
5 REGULATIONS OF EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET
6 EPIDEMIOLOGY OF DISEASES
6.1 INCIDENCE OF ISCHEMIA
6.2 INCIDENCE OF PARKINSON'S DISEASE
6.3 INCIDENCE OF FAILED BACK SYNDROME
6.4 INCIDENCE OF TREMOR
6.5 INCIDENCE OF DEPRESSION
6.6 INCIDENCE OF URINE INCONTINENCE
6.7 INCIDENCE OF FECAL INCONTINENCE
6.8 INCIDENCE RATE OF EPILEPSY
6.9 INCIDENCE OF GASTROPARESIS
6.1 PREVALENCE OF OBESITY
7 EPIDEMIOLOGY OF NEUROSTIMULATION PROCEDURES
7.1 NUMBER OF SPINAL CORD STIMULATION (SCS) PROCEDURES
7.1.1 NUMBER OF TEST PROCEDURES
7.1.2 NUMBER OF IMPLANTATION PROCEDURES
7.2 NUMBER OF DEEP BRAIN STIMULATION PROCEDURES
7.3 NUMBER OF VAGUS NERVE STIMULATION PROCEDURES
7.4 NUMBER OF SACRAL NEVER STIMULATION PROCEDURES
7.5 NUMBER OF TRANSCRANIAL MAGNETIC STIMULATION (TMS) PROCEDURES
7.6 NUMBER OF INTERMITTENT THETA BURST STIMULATION (ITBS) PROCEDURES
7.7 NUMBER OF TRANSCRANIAL DIRECT ELECTRICAL STIMULATION (TDCS) PROCEDURES
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 RISE IN PREVALENCE AND INCIDENCE OF NEUROLOGICAL DISORDERS
8.1.2 DEMAND FOR INTERNAL NEUROSTIMULATION DEVICES AS A ADD ON THERAPY
8.1.3 INCREASED FUNDING FOR THE NEUROSTIMULATION DEVICES
8.1.4 TECHNOLOGICAL ADVANCEMENTS IN THE INTERNAL NEUROSTIMULATION DEVICES
8.1.5 RISE IN PRODUCT APPROVALS
8.2 RESTRAINTS
8.2.1 RISE IN COST OF THE DEEP BRAIN STIMULATION DEVICES
8.2.2 RISKS NOTICED WHILE USING THE INTERNAL NEUROSTIMULATION DEVICES
8.2.3 RISE IN PRODUCT RECALL
8.2.4 AVAILABILITY OF ALTERNATE IMAGING DIAGNOSTIC DEVICES
8.3 OPPORTUNITIES
8.3.1 STRATEGIC INITIATIVES BY THE KEY MARKET PLAYER
8.3.2 RECENT PRODUCT DEVELOPMENTS IN THE INTERNAL NEUROSTIMULATION DEVICES
8.3.3 DEMAND FOR MINIMALLY INVASIVE SURGERY
8.4 CHALLENGES
8.4.1 RISKS ASSOCIATED WITH THE IMPLANTATION OF THESE DEVICES
8.4.2 LACK OF SKILLED HEALTHCARE PROFESSIONALS
9 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE
9.1 OVERVIEW
9.2 SPINAL CORD STIMULATION
9.2.1 SPINAL CORD STIMULATION, BY TYPE
9.2.1.1 BATTERY
9.2.1.1.1 RECHARGEABLE
9.2.1.1.2 NON-RECHARGEABLE
9.2.1.2 LEAD
9.2.1.2.1 PERCUTANEOUS
9.2.1.2.2 PADDLE
9.2.2 SPINAL CORD STIMULATION, BY APPLICATION
9.2.2.1 ISCHEMIA
9.2.2.2 CHRONIC LOW BACK PAIN (CLBP)
9.2.2.3 DIABETIC NEUROPATHY
9.2.2.4 FAILED BACK SYNDROME
9.3 DEEP BRAIN STIMULATION
9.3.1 DEEP BRAIN STIMULATION, BY TYPE
9.3.1.1 SINGLE CHANNEL DEEP BRAIN STIMULATOR
9.3.1.2 DOUBLE CHANNEL DEEP BRAIN STIMULATOR
9.3.1.3 BATTERY
9.3.1.3.1 RECHARGEABLE
9.3.1.3.2 NON-RECHARGEABLE
9.3.1.4 LEAD
9.3.2 DEEP BRAIN STIMULATION, BY APPLICATION
9.3.2.1 PARKINSON’S DISEASE
9.3.2.2 TREMOR
9.3.2.3 DEPRESSION
9.4 SACRAL NERVE STIMULATION
9.4.1 SACRAL NERVE STIMULATION, BY TYPE
9.4.1.1 BATTERY
9.4.1.2 LEAD
9.5 SACRAL NERVE STIMULATION, BY APPLICATION
9.5.1 URINE INCONTINENCE
9.5.2 FECAL INCONTINENCE
9.6 VAGUS NERVE STIMULATION
9.6.1 VAGUS NERVE STIMULATION, BY TYPE
9.6.1.1 BATTERY
9.6.1.2 LEAD
9.6.2 VAGUS NERVE STIMULATION, BY APPLICATION
9.6.2.1 EPILEPSY
9.6.2.2 OTHERS
9.7 GASTRIC ELECTRICAL STIMULATION
9.7.1 GASTRIC ELECTRICAL STIMULATION, BY TYPE
9.7.1.1 BATTERY
9.7.1.2 LEAD
9.7.2 GASTRIC ELECTRICAL STIMULATION, BY APPLICATION
9.7.2.1 GASTROPARESIS
9.7.2.2 OTHERS
10 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL
10.1 OVERVIEW
10.2 DIRECT TENDER
10.3 THIRD PARTY PROVIDER
11 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET, BY REGION
11.1 EUROPE
11.1.1 GERMANY
11.1.2 U.K.
11.1.3 ITALY
11.1.4 SPAIN
11.1.5 FRANCE
11.1.6 SWITZERLAND
11.1.7 BELGIUM
11.1.8 NETHERLANDS
11.1.9 RUSSIA
11.1.10 TURKEY
11.1.11 REST OF EUROPE
12 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: COMPANY LANDSCAPE
12.1 COMPANY SHARE ANALYSIS: EUROPE
13 SWOT ANALYSIS
14 COMPANY PROFILE
14.1 MEDTRONIC (2021)
14.1.1 COMPANY SNAPSHOT
14.1.2 REVENUE ANALYSIS
14.1.3 COMPANY SHARE ANALYSIS
14.1.4 PRODUCT PORTFOLIO
14.1.5 RECENT DEVELOPMENTS
14.2 BOSTON SCIENTIFIC CORPORATION (2021)
14.2.1 COMPANY SNAPSHOT
14.2.2 REVENUE ANALYSIS
14.2.3 COMPANY SHARE ANALYSIS
14.2.4 PRODUCT PORTFOLIO
14.2.5 RECENT DEVELOPMENTS
14.3 ABBOTT (2021)
14.3.1 COMPANY SNAPSHOT
14.3.2 REVENUE ANALYSIS
14.3.3 COMPANY SHARE ANALYSIS
14.3.4 PRODUCT PORTFOLIO
14.3.5 RECENT DEVELOPMENTS
14.4 LIVANOVA PLC (2021)
14.4.1 COMPANY SNAPSHOT
14.4.2 REVENUE ANALYSIS
14.4.3 COMPANY SHARE ANALYSIS
14.4.4 PRODUCT PORTFOLIO
14.4.5 RECENT DEVELOPMENTS
14.5 NEVRO CORP. (2021)
14.5.1 COMPANY SNAPSHOT
14.5.2 REVENUE ANALYSIS
14.5.3 COMPANY SHARE ANALYSIS
14.5.4 PRODUCT PORTFOLIO
14.5.5 RECENT DEVELOPMENTS
14.6 AXONICS, INC.
14.6.1 COMPANY SNAPSHOT
14.6.2 REVENUE ANALYSIS
14.6.3 PRODUCT PORTFOLIO
14.6.4 RECENT DEVELOPMENTS
14.7 ALEVA NEUROTHERAPEUTICS
14.7.1 COMPANY SNAPSHOT
14.7.2 PRODUCT PORTFOLIO
14.7.3 RECENT DEVELOPMENTS
14.8 BIONIC VISION TECHNOLOGIES.
14.8.1 COMPANY SNAPSHOT
14.8.2 PRODUCT PORTFOLIO
14.8.3 RECENT DEVELOPMENTS
14.9 BLUEWIND MEDICAL
14.9.1 COMPANY SNAPSHOT
14.9.2 PRODUCT PORTFOLIO
14.9.3 RECENT DEVELOPMENTS
14.1 BIOINDUCTION
14.10.1 COMPANY SNAPSHOT
14.10.2 PRODUCT PORTFOLIO
14.10.3 RECENT DEVELOPMENT
14.11 CIRTEC
14.11.1 COMPANY SNAPSHOT
14.11.2 PRODUCT PORTFOLIO
14.11.3 RECENT DEVELOPMENTS
14.12 FINETECH MEDICAL
14.12.1 COMPANY SNAPSHOT
14.12.2 PRODUCT PORTFOLIO
14.12.3 RECENT DEVELOPMENTS
14.13 GIMER MEDICAL
14.13.1 COMPANY SNAPSHOT
14.13.2 PRODUCT PORTFOLIO
14.13.3 RECENT DEVELOPMENTS
14.14 INSPIRE MEDICAL SYSTEMS, INC.
14.14.1 COMPANY SNAPSHOT
14.14.2 REVENUE ANALYSIS
14.14.3 PRODUCT PORTFOLIO
14.14.4 RECENT DEVELOPMENTS
14.15 INTEGER HOLDINGS CORPORATION
14.15.1 COMPANY SNAPSHOT
14.15.2 REVENUE ANALYSIS
14.15.3 PRODUCT PORTFOLIO
14.15.4 RECENT DEVELOPMENT
14.16 INBRAIN NEUROELECTRONICS
14.16.1 COMPANY SNAPSHOT
14.16.2 PRODUCT PORTFOLIO
14.16.3 RECENT DEVELOPMENT
14.17 MAINSTAY MEDICAL
14.17.1 COMPANY SNAPSHOT
14.17.2 REVENUE ANALYSIS
14.17.3 PRODUCT PORTFOLIO
14.17.4 RECENT DEVELOPMENTS
14.18 MICROSEMI (A WHOLLY OWNDED SUBSIDIARY OF MICROCHIP TECHNOLOGY INC.)
14.18.1 COMPANY SNAPSHOT
14.18.2 REVENUE ANALYSIS
14.18.3 PRODUCT PORTFOLIO
14.18.4 RECENT DEVELOPMENTS
14.19 MICROTRANSPONDER INC
14.19.1 COMPANY SNAPSHOT
14.19.2 PRODUCT PORTFOLIO
14.19.3 RECENT DEVELOPMENT
14.2 MICRO-LEADS
14.20.1 COMPANY SNAPSHOT
14.20.2 PRODUCT PORTFOLIO
14.20.3 RECENT DEVELOPMENT
14.21 NALU MEDICAL, INC
14.21.1 COMPANY SNAPSHOT
14.21.2 PRODUCT PORTFOLIO
14.21.3 RECENT DEVELOPMENT
14.22 NEURONANO AB
14.22.1 COMPANY SNAPSHOT
14.22.2 PRODUCT PORTFOLIO
14.22.3 RECENT DEVELOPMENTS
14.23 NEURIMPULSE S.R.L.
14.23.1 COMPANY SNAPSHOT
14.23.2 PRODUCT PORTFOLIO
14.23.3 RECENT DEVELOPMENTS
14.24 NEWRONIKA S.P.A.
14.24.1 COMPANY SNAPSHOT
14.24.2 PRODUCT PORTFOLIO
14.24.3 RECENT DEVELOPMENTS
14.25 OPTOGENTECH GMBH.
14.25.1 COMPANY SNAPSHOT
14.25.2 PRODUCT PORTFOLIO
14.25.3 RECENT DEVELOPMENTS
14.26 ONWARD
14.26.1 COMPANY SNAPSHOT
14.26.2 PRODUCT PORTFOLIO
14.26.3 RECENT DEVELOPMENT
14.27 STIMWAVE LLC (2021)
14.27.1 COMPANY SNAPSHOT
14.27.2 PRODUCT PORTFOLIO
14.27.3 RECENT DEVELOPMENTS
14.28 SEQUANA MEDICAL NV (2021)
14.28.1 COMPANY SNAPSHOT
14.28.2 REVENUE ANALYSIS
14.28.3 PRODUCT PORTFOLIO
14.28.4 RECENT DEVELOPMENTS
14.29 VALENCIA TECHNOLOGIES
14.29.1 COMPANY SNAPSHOT
14.29.2 PRODUCT PORTFOLIO
14.29.3 RECENT DEVELOPMENTS
15 QUESTIONNAIRE
16 RELATED REPORTS
Lista de Tablas
TABLE 1 EUROPE PREVALENCE OF OBESITY
TABLE 2 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 3 EUROPE SPINAL CORD STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 EUROPE SPINAL CORD STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 5 EUROPE BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 6 EUROPE BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 7 EUROPE BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 8 EUROPE LEAD IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 9 EUROPE LEAD IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 10 EUROPE LEAD IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 11 EUROPE SPINAL CORD STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 12 EUROPE DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, 2020-2029 (USD MILLION)
TABLE 13 EUROPE DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 14 EUROPE DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 15 EUROPE DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 16 EUROPE BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 17 EUROPE BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 18 EUROPE BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 19 EUROPE DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 20 EUROPE SACRAL NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, 2020-2029 (USD MILLION)
TABLE 21 EUROPE SACRAL NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 22 EUROPE SACRAL NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 23 EUROPE SACRAL NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 24 EUROPE SACRAL NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 25 EUROPE VAGUS NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, 2020-2029 (USD MILLION)
TABLE 26 EUROPE VAGUS NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 27 EUROPE VAGUS NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 28 EUROPE VAGUS NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 29 EUROPE VAGUS NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 30 EUROPE GASTRIC ELECTRICAL STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, 2020-2029 (USD MILLION)
TABLE 31 EUROPE GASTRIC ELECTRICAL STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 32 EUROPE GASTRIC ELECTRICAL STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 33 EUROPE GASTRIC ELECTRICAL STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 34 EUROPE GASTRIC ELECTRICAL STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 35 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 36 EUROPE DIRECT TENDER IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 37 EUROPE THIRD PARTY PROVIDER IN INTERNAL NEUROSTIMULATION DEVICES MARKET, 2020-2029 (USD MILLION)
TABLE 38 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 39 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 40 EUROPE SPINAL CORD STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 41 EUROPE BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 42 EUROPE BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 43 EUROPE BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 44 EUROPE LEAD IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 45 EUROPE LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 46 EUROPE LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 47 EUROPE SPINAL CORD STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 48 EUROPE DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 49 EUROPE DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 50 EUROPE DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 51 EUROPE BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 52 EUROPE BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 53 EUROPE BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 54 EUROPE DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 55 EUROPE SACRAL NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 56 EUROPE SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 57 EUROPE SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 58 EUROPE SACRAL NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 59 EUROPE VAGUS NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 60 EUROPE VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 61 EUROPE VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 62 EUROPE VAGUS NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 63 EUROPE GASTRIC ELECTRICAL STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 64 EUROPE GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 65 EUROPE GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 66 EUROPE GASTRIC ELECTRICAL STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 67 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 68 GERMANY INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 69 GERMANY SPINAL CORD STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 70 GERMANY BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 71 GERMANY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 72 GERMANY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 73 GERMANY LEAD IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 74 GERMANY LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 75 GERMANY LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 76 GERMANY SPINAL CORD STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 77 GERMANY DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 78 GERMANY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 79 GERMANY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 80 GERMANY BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 81 GERMANY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 82 GERMANY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 83 GERMANY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 84 GERMANY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 85 GERMANY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 86 GERMANY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 87 GERMANY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 88 GERMANY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 89 GERMANY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 90 GERMANY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 91 GERMANY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 92 GERMANY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 93 GERMANY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 94 GERMANY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 95 GERMANY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 96 GERMANY NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 97 U.K. NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 98 U.K. SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 99 U.K. BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 100 U.K. BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 101 U.K. BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 102 U.K. LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 103 U.K. LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 104 U.K. LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 105 U.K. SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 106 U.K. DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 107 U.K. DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 108 U.K. DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 109 U.K. BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 110 U.K. BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 111 U.K. BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 112 U.K. DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 113 U.K. SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 114 U.K. SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 115 U.K. SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 116 U.K. SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 117 U.K. VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 118 U.K. VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 119 U.K. VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 120 U.K. VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 121 U.K. GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 122 U.K. GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 123 U.K. GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 124 U.K. GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 125 ITALY NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 126 ITALY SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 127 ITALY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 128 ITALY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 129 ITALY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 130 ITALY LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 131 ITALY LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 132 ITALY LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 133 ITALY SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 134 ITALY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 135 ITALY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 136 ITALY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 137 ITALY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 138 ITALY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 139 ITALY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 140 ITALY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 141 ITALY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 142 ITALY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 143 ITALY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 144 ITALY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 145 ITALY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 146 ITALY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 147 ITALY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 148 ITALY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 149 ITALY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 150 ITALY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 151 ITALY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 152 ITALY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 153 ITALY NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 154 SPAIN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 155 SPAIN SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 156 SPAIN BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 157 SPAIN BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 158 SPAIN BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 159 SPAIN LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 160 SPAIN LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 161 SPAIN LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 162 SPAIN SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 163 SPAIN DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 164 SPAIN DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 165 SPAIN DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 166 SPAIN BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 167 SPAIN BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 168 SPAIN BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 169 SPAIN DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 170 SPAIN SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 171 SPAIN SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 172 SPAIN SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 173 SPAIN SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 174 SPAIN VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 175 SPAIN VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 176 SPAIN VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 177 SPAIN VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 178 SPAIN GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 179 SPAIN GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 180 SPAIN GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 181 SPAIN GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 182 SPAIN NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 183 FRANCE NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 184 FRANCE SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 185 FRANCE BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 186 FRANCE BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 187 FRANCE BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 188 FRANCE LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 189 FRANCE LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 190 FRANCE LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 191 FRANCE SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 192 FRANCE DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 193 FRANCE DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 194 FRANCE DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 195 FRANCE BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 196 FRANCE BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 197 FRANCE BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 198 FRANCE DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 199 FRANCE SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 200 FRANCE SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 201 FRANCE SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 202 FRANCE SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 203 FRANCE VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 204 FRANCE VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 205 FRANCE VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 206 FRANCE VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 207 FRANCE GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 208 FRANCE GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 209 FRANCE GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 210 FRANCE GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 211 FRANCE NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 212 SWITZERLAND NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 213 SWITZERLAND SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 214 SWITZERLAND BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 215 SWITZERLAND BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 216 SWITZERLAND BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 217 SWITZERLAND LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 218 SWITZERLAND LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 219 SWITZERLAND LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 220 SWITZERLAND SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 221 SWITZERLAND DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 222 SWITZERLAND DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 223 SWITZERLAND DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 224 SWITZERLAND BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 225 SWITZERLAND BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 226 SWITZERLAND BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 227 SWITZERLAND DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 228 SWITZERLAND SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 229 SWITZERLAND SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 230 SWITZERLAND SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 231 SWITZERLAND SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 232 SWITZERLAND VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 233 SWITZERLAND VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 234 SWITZERLAND VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 235 SWITZERLAND VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 236 SWITZERLAND GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 237 SWITZERLAND GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 238 SWITZERLAND GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 239 SWITZERLAND GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 240 SWITZERLAND NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 241 BELGIUM NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 242 BELGIUM SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 243 BELGIUM BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 244 BELGIUM BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 245 BELGIUM BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 246 BELGIUM LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 247 BELGIUM LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 248 BELGIUM LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 249 BELGIUM SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 250 BELGIUM DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 251 BELGIUM DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 252 BELGIUM DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 253 BELGIUM BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 254 BELGIUM BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 255 BELGIUM BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 256 BELGIUM DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 257 BELGIUM SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 258 BELGIUM SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 259 BELGIUM SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 260 BELGIUM SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 261 BELGIUM VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 262 BELGIUM VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 263 BELGIUM VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 264 BELGIUM VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 265 BELGIUM GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 266 BELGIUM GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 267 BELGIUM GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 268 BELGIUM GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 269 BELGIUM NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 270 NETHERLANDS NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 271 NETHERLANDS SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 272 NETHERLANDS BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 273 NETHERLANDS BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 274 NETHERLANDS BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 275 NETHERLANDS LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 276 NETHERLANDS LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 277 NETHERLANDS LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 278 NETHERLANDS SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 279 NETHERLANDS DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 280 NETHERLANDS DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 281 NETHERLANDS DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 282 NETHERLANDS BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 283 NETHERLANDS BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 284 NETHERLANDS BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 285 NETHERLANDS DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 286 NETHERLANDS SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 287 NETHERLANDS SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 288 NETHERLANDS SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 289 NETHERLANDS SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 290 NETHERLANDS VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 291 NETHERLANDS VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 292 NETHERLANDS VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 293 NETHERLANDS VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 294 NETHERLANDS GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 295 NETHERLANDS GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 296 NETHERLANDS GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 297 NETHERLANDS GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 298 NETHERLANDS NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 299 RUSSIA NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 300 RUSSIA SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 301 RUSSIA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 302 RUSSIA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 303 RUSSIA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 304 RUSSIA LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 305 RUSSIA LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 306 RUSSIA LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 307 RUSSIA SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 308 RUSSIA DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 309 RUSSIA DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)-
TABLE 310 RUSSIA DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 311 RUSSIA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 312 RUSSIA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 313 RUSSIA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 314 RUSSIA DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 315 RUSSIA SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 316 RUSSIA SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 317 RUSSIA SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 318 RUSSIA SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 319 RUSSIA VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 320 RUSSIA VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 321 RUSSIA VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 322 RUSSIA VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 323 RUSSIA GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 324 RUSSIA GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 325 RUSSIA GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 326 RUSSIA GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 327 RUSSIA NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 328 TURKEY NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 329 TURKEY SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 330 TURKEY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 331 TURKEY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 332 TURKEY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 333 TURKEY LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 334 TURKEY LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 335 TURKEY LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 336 TURKEY SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 337 TURKEY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 338 TURKEY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 339 TURKEY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 340 TURKEY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 341 TURKEY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 342 TURKEY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 343 TURKEY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 344 TURKEY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 345 TURKEY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 346 TURKEY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 347 TURKEY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 348 TURKEY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 349 TURKEY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 350 TURKEY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 351 TURKEY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 352 TURKEY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 353 TURKEY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 354 TURKEY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 355 TURKEY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 356 TURKEY NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 357 REST OF EUROPE NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
Lista de figuras
FIGURE 1 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: SEGMENTATION
FIGURE 2 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: GEOGRAPHIC SCOPE
FIGURE 3 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: DATA TRIANGULATION
FIGURE 4 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: SNAPSHOT
FIGURE 5 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BOTTOM UP APPROACH
FIGURE 6 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: TOP DOWN APPROACH
FIGURE 7 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: INTERVIEWS BY REGION AND DESIGNATION
FIGURE 8 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: DBMR MARKET POSITION GRID
FIGURE 9 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: THE CATEGORY VS TIME GRID
FIGURE 10 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET SEGMENTATION
FIGURE 11 INCREASE IN PREVALENCE AND INCIDENCE OF NEUROLOGICAL DISEASES AND DEMAND FOR INTERNAL NEUROSTIMULATION DEVICES AS A ADD ON THERAPY ARE EXPECTED TO DRIVE THE EUROPE INTERNAL NEUROSTIMULATIOJ DEVICES MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 SPINAL CORD STIMULATION IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET IN 2022 TO 2029
FIGURE 13 EUROPE INCIDENCE OF ISCHEMIA
FIGURE 14 EUROPE INCIDENCE OF PARKINSON'S DISEASES
FIGURE 15 EUROPE INCIDENCE OF TREMOR
FIGURE 16 EUROPE INCIDENCE RATE OF EPILEPSY
FIGURE 17 EUROPE INCIDENCE RATE OF GASTROPARESIS
FIGURE 18 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET
FIGURE 19 INCIDENCE OF ADULT ONSET BRAIN DISORDERS IN THE U.S. IN 2021
FIGURE 20 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY PRODUCT TYPE, 2021
FIGURE 21 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY PRODUCT TYPE, 2022-2029 (USD MILLION)
FIGURE 22 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY PRODUCT TYPE, CAGR (2022-2029)
FIGURE 23 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 24 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 25 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 26 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 27 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 28 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: SNAPSHOT (2021)
FIGURE 29 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY COUNTRY (2021)
FIGURE 30 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY COUNTRY (2022 & 2029)
FIGURE 31 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY COUNTRY (2021 & 2029)
FIGURE 32 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY PRODUCT TYPE (2022-2029)
FIGURE 33 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: COMPANY SHARE 2021 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.













