中東およびアフリカの遺伝子検査市場、タイプ別(保因者検査、診断検査、新生児スクリーニング、予測および発症前検査、出生前検査、その他のタイプ)、技術別(DNAシーケンシング(NGSベースの検査)、ポリメラーゼ連鎖反応、マイクロアレイ、全ゲノムシーケンシング、蛍光in situハイブリダイゼーション(FISH)、その他)、疾患別(希少遺伝性疾患、がん、嚢胞性線維症、生殖遺伝子検査、健康とウェルネス遺伝子検査、鎌状赤血球貧血、デュシェンヌ型筋ジストロフィー、サラセミア、ハンチントン病、脆弱X症候群、その他)、エンドユーザー別(病院、診療所、診断センター、民間診療所、検査サービスプロバイダー、民間研究所)業界動向と2030年までの予測。
中東およびアフリカの遺伝子検査市場の分析と洞察
中東およびアフリカの遺伝子検査市場は、遺伝性疾患の有病率の高さ、遺伝子検査市場における技術進歩の進展による需要の増大、市場の成長につながる研究開発への投資増加などの要因によって牽引されています。現在、先進国および新興国全体で医療費が増加しており、これによりメーカーが新しい革新的な遺伝子検査市場を開発するための競争上の優位性が生まれると予想されています。
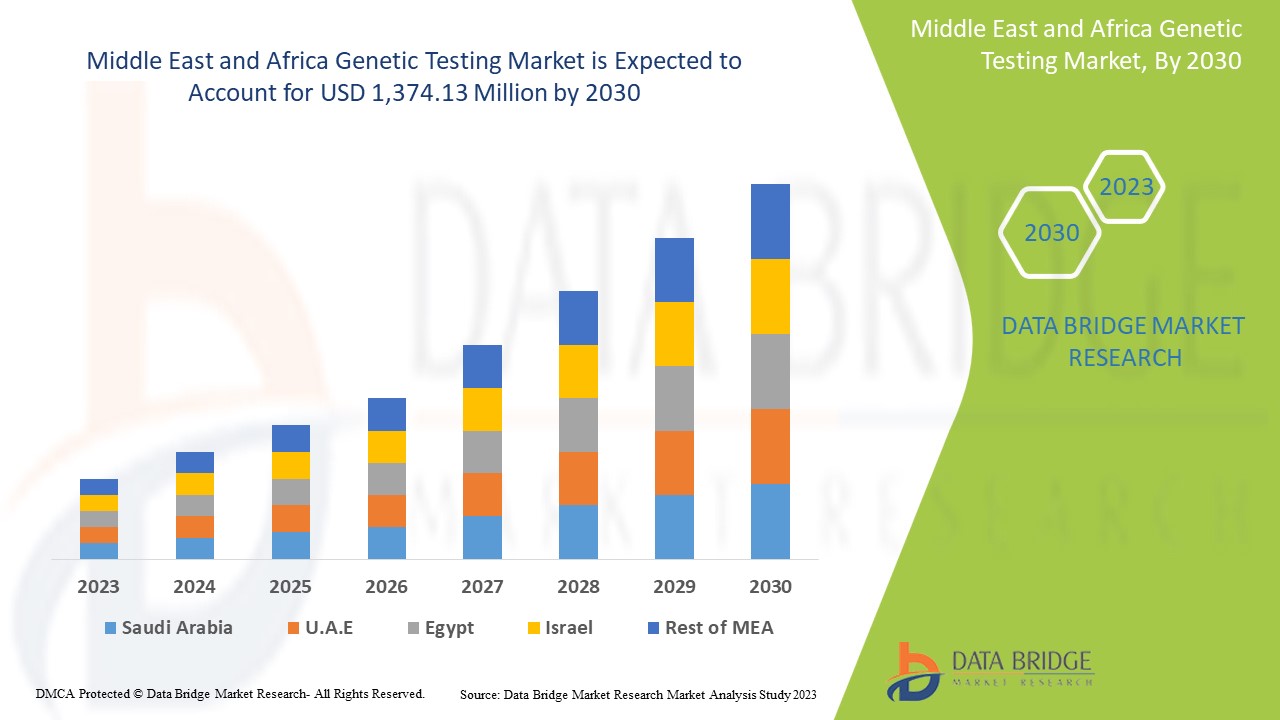
中東およびアフリカの遺伝子検査市場は、2023年から2030年の予測期間に市場成長が見込まれています。データブリッジマーケットリサーチは、市場は2023年から2030年の予測期間に13.6%のCAGRで成長し、2030年までに13億7,413万米ドルに達すると分析しています。
中東およびアフリカの遺伝子検査市場レポートでは、市場シェア、新開発、製品パイプライン分析、国内および現地の市場プレーヤーの影響、新たな収益源、市場規制の変更、製品承認、戦略的決定、製品発売、地理的拡大、市場における技術革新の観点から見た機会の詳細が提供されます。分析と市場シナリオを理解するには、アナリスト概要についてお問い合わせください。当社のチームが、収益に影響を与えるソリューションを作成し、希望する目標を達成できるようお手伝いします。
|
レポートメトリック |
詳細 |
|
予測期間 |
2023年から2030年 |
|
基準年 |
2022 |
|
歴史的な年 |
2021 (2020~2015年にカスタマイズ可能) |
|
定量単位 |
収益(百万米ドル) |
|
対象セグメント |
タイプ別 (保因者検査、診断検査、新生児スクリーニング、予測および発症前検査、出生前検査、その他のタイプ)、技術別 (DNA シーケンシング (NGS ベースの検査)、ポリメラーゼ連鎖反応、マイクロアレイ、全ゲノムシーケンシング、蛍光 in situ ハイブリダイゼーション (FISH)、その他)、疾患別 (希少遺伝性疾患、がん、嚢胞性線維症、生殖遺伝子検査、健康とウェルネス遺伝子検査、鎌状赤血球貧血、デュシェンヌ型筋ジストロフィー、サラセミア、ハンチントン病、脆弱 X 症候群、その他)、エンドユーザー別 (病院、診療所、診断センター、民間診療所、検査サービス プロバイダー、民間研究所)。 |
|
対象国 |
南アフリカ、サウジアラビア、UAE、エジプト、イスラエル、その他の中東およびアフリカ。 |
|
対象となる市場プレーヤー |
Abbott、23ANDME、Inc.、Danaher、Myriad Genetics、Inc.、OCP Medical Centre、LLC、Healthchecks360、Bayer AG、BioReference Health、LLC(OPKO Health、Inc.の子会社)、FREIBURG MEDICAL LABORATORY MIDDLE EAST(LLC)、Thermo Fisher Scientific Inc.、Bio-Rad Laboratories、Inc.、Biocartis、Eurofins Scientific、PerkinElmer Inc.、ELITechGroup、F. Hoffmann-La Roche Ltd.、Illumina、Inc.、QIAGEN、BIO-HELIX、PacBio など。 |
市場の定義
遺伝子検査は、遺伝子、染色体、またはタンパク質の変化を特定する医療検査の一種です。遺伝子検査の結果は、疑わしい遺伝的疾患を確認または除外したり、遺伝性疾患を発症したり遺伝したりする可能性を判断するのに役立ちます。現在 77,000 を超える遺伝子検査が使用されており、さらに新しい検査が開発されています。
革新と技術の増加、市場参入者数の増加、そして斬新な製品の発売も、中東およびアフリカの遺伝子検査市場の成長を促進しています。
中東およびアフリカの遺伝子検査市場の動向
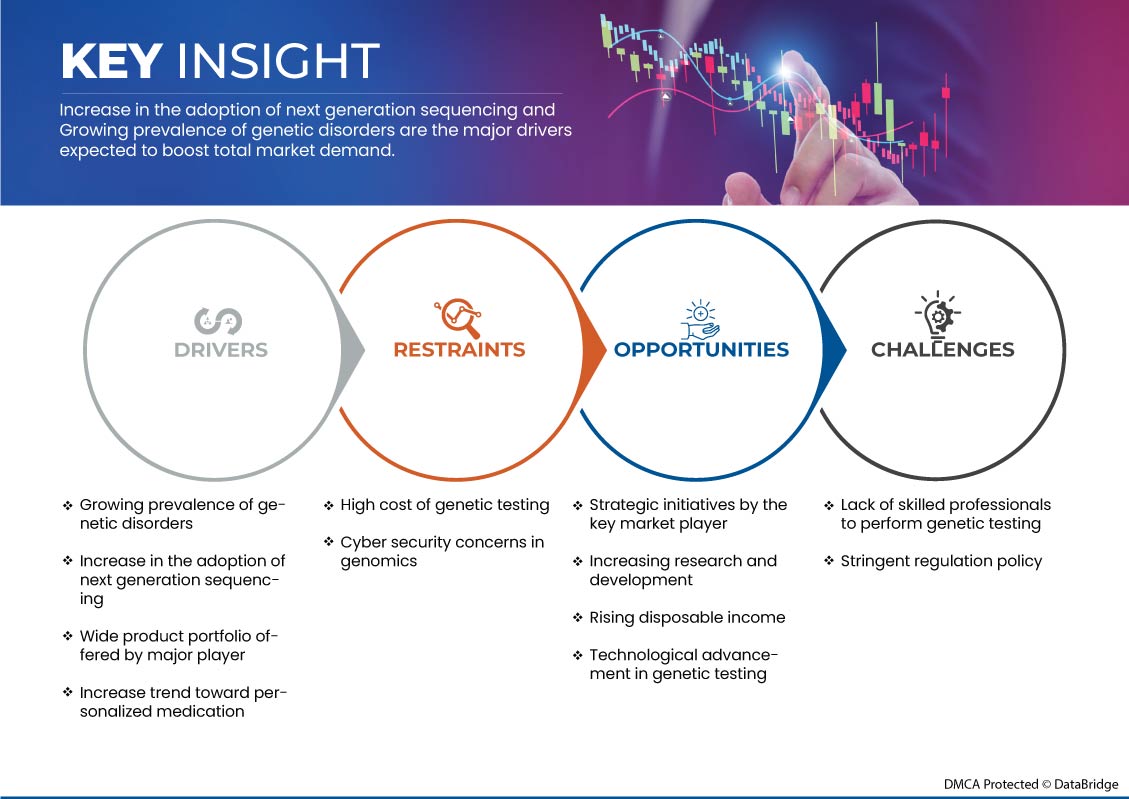
このセクションでは、市場の推進要因、利点、機会、制約、課題について理解します。これらについては、以下で詳しく説明します。
推進要因/機会
- 遺伝性疾患の増加
遺伝性疾患は、生命維持に適さないほどの深刻な健康問題を引き起こす可能性があります。最も深刻なケースでは、これらの疾患は影響を受けた胚または胎児の流産を引き起こす可能性があります。遺伝性疾患や先天性欠損症の蔓延により、遺伝子検査市場の需要が高まっています。
- 世界保健機関の「遺伝性疾患と先天異常:地域における負担軽減戦略 2022」の記事によると、遺伝性疾患と先天性疾患は、この地域の多くの国で周産期および新生児死亡率のかなりの部分を占めています。出生異常は現在、アラブ首長国連邦では乳児死亡率の第一の原因とされ、バーレーン、クウェート、オマーン、カタールでは第二の原因となっています。サウジアラビアからの報告によると、2つの病院での出生前死亡の約25~35%は出生異常によるものでした。
したがって、遺伝子検査市場の需要が高まっています。
- 次世代シーケンシングの採用の増加
ゲノミクスに焦点を当てた薬理学がさまざまな慢性疾患、特に癌の治療において大きな役割を果たし続けるにつれて、次世代シーケンシング (NGS) は、個々の腫瘍や特定の受容体の分子基盤に対するより深く正確な洞察を提供する強力なツールとして進化しています。
NGS は、従来の方法に比べて精度、感度、速度の面で優れており、腫瘍学の分野に大きな影響を与える可能性があります。NGS では 1 回のアッセイで複数の遺伝子を評価できるため、原因となる変異を特定するために複数のテストを注文する必要がなくなります。
したがって、これが遺伝子検査市場の成長の原動力となることが期待されます。
- 可処分所得の増加
国が医療に費やす費用とその長期にわたる成長率は、資金調達の取り決めや医療制度の組織構造など、さまざまな経済的および社会的要因によって左右されます。特に、国の総所得レベルとその国の国民が医療に費やす金額の間には強い関連性があります。
また、主要な市場プレーヤーが講じる戦略的取り組みは、2023~2030年の予測期間において、遺伝子検査市場に構造的完全性と将来の機会をもたらすでしょう。
制約/課題
- 遺伝子検査の高額な費用
遺伝子検査は高額になる可能性があり、一部の健康保険ではカバーされない場合があります。遺伝子検査は数多くありますが、検査対象となる疾患によって費用が異なります。
- Breastcancer.orgによると、がんの遺伝子検査の費用は大きく異なり、300ドルから5,000ドルの間になる可能性がある。遺伝子検査の費用は、検査の種類と複雑さによって異なる。
- 遺伝子検査の費用は、検査の性質や複雑さに応じて、100ドルから2,000ドル以上までさまざまです。複数の検査が必要な場合や、有意な結果を得るために多数の家族を検査する必要がある場合は、費用が高くなります。新生児スクリーニングの費用は州によって異なります。
したがって、遺伝子検査のコストが高いことが市場の成長を抑制する可能性があります。
- 厳格な規制政策
規制業務 (RA) は、医療機器業界ではヘルスケア製品のライフサイクルに関わるため、重要な役割を果たします。RA は、戦術的、戦略的、運用的な道筋を提供し、規制の範囲内で機能して、安全かつ効果的な癌唾液検査機器および製品を世界中の人々に迅速に提供および開発するのを支援します。規制業務の役割は、規制戦略を確立して実行することです。
Many healthcare organizations and government agencies are providing regulatory policies for the launch and approval of cancer spit test devices and products. Approval for the product by regional regulators plays an essential role for the drug development team. The approval assures the company's drug development activities and differentiates the company from the competition.
Post-COVID-19 Impact on the Middle East and Africa Genetic Testing Market
The genetic testing market has been badly affected by COVID-19. Hospital admissions were limited to non-essential treatment, and clinics were temporarily closed during the pandemic. The implementation of social distancing, blocking the population, and limited access to clinics have greatly affected the market. The slowdown in patient flows and referrals also affected the market growth. However, the market will continue to grow in the post-pandemic period due to the relaxation of previously imposed restrictions.
Manufacturers are making various strategic decisions to bounce back post-COVID-19. The players are conducting multiple R&D activities and product launches, and strategic partnerships to improve the technology and test results involved in the genetic testing market.
Recent Development
- On 10 November 2022, Myriad Genetics, Inc., a leader in genetic testing and precision medicine, announced UroSuite, a comprehensive suite of genetic risk assessment tests that cover all stages of prostate cancer care. UroSuite includes Myriad's Prolaris Prostate Cancer Test, MyRisk Hereditary Cancer Test, BRACAnalysis CDx, and Precise Tumor Molecular Profile Test. The combination of tests provides an integrated genetic overview and facilitates treatment and selection of clinical trials for patients.
- In September 2022, Illumina, Inc., a world leader in DNA sequencing and array-based technologies, announced the launch of the NovaSeq X-Series (NovaSeq X and NovaSeq X Plus). These are new production-scale sequencers that push the boundaries of genomic medicine by enabling faster, more efficient, and more sustainable sequencing. With revolutionary new technology, NovaSeq X Plus can generate more than 20,000 whole genomes per year—2.5 times the performance of previous sequencers—dramatically accelerating genome discovery and clinical insights to understand the disease and ultimately transform patients' lives.
Middle East and Africa Genetic Testing Market Scope
Middle East and Africa genetic testing market is segmented into type, technology, diseases, and end user. The growth amongst these segments will help you analyze and measure growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
Type
- Diagnostic testing
- Prenatal testing
- New born screening
- Predictive and presymptomatic testing
- Carrier testing
- Other types
On the basis of type, the Middle East and Africa genetic testing market is segmented into diagnostic testing, prenatal testing, new born screening, predictive and presymptomatic testing, carrier testing other types.
TECHNOLOGY
- Polymerase chain reaction
- DNA sequencing (NGS-based testing)
- Whole genome sequencing
- Microarrays
- Fluorescence in situ hybridization (FISH)
- Others
On the basis of technology, the Middle East and Africa genetic testing market is segmented into DNA sequencing, polymerase chain reaction, microarrays, whole genome sequencing, fluorescence in situ hybridization (FISH), and others.
Diseases
- Cancer
- Sickle cell anemia
- Thalassemia
- Rare genetic disorder
- Fragile X Syndrome
- Duchenne muscular dystrophy
- Huntington's disease
- Cystic Fibrosis
- Reproductive genetic testing
- Health & wellness genetic testing
- Others
On the basis of diseases, the Middle East and Africa genetic testing market is segmented into rare Genetic Disorder, cancer, cystic fibrosis, sickle cell anemia, Duchenne muscular dystrophy, thalassemia, Huntington's disease, fragile X syndrome, reproductive genetic testing, health & wellness genetic testing, and other.
End User
- Hospitals
- Clinics
- Diagnostic centers
- Private clinics
- Laboratory service providers
- Private laboratories
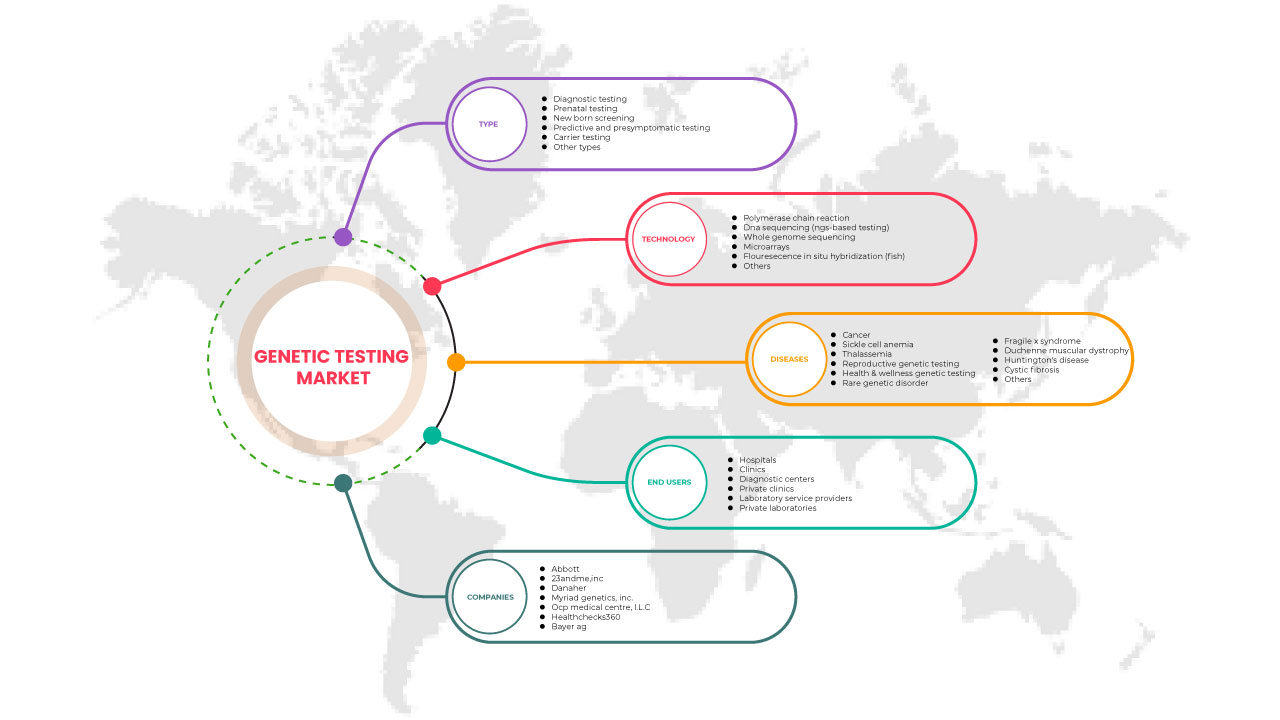
On the basis of end users, the Middle East and Africa genetic testing market is segmented into hospitals, clinics, diagnostic centers, private clinics, laboratory service providers, and private laboratories.
Middle East and Africa Genetic Testing Market Regional Analysis/Insights
The Middle East and Africa genetic testing market is analyzed, and market size information is provided by type, technology, diseases, and end-user.
The countries covered in this market report are South Africa, Saudi Arabia, UAE, Egypt, Israel, the rest of the Middle East and Africa.
In 2023, South Africa is expected to dominate due to the increasing investment in R&D is expected to boost the market growth.
The country section of the report also provides individual market-impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of Middle East and Africa brands and their challenges faced due to large or scarce competition from local and domestic brands and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Middle East and Africa Genetic Testing Market Share Analysis
中東およびアフリカの遺伝子検査市場の競争環境は、競合他社によって詳細が提供されています。詳細には、会社概要、会社の財務状況、収益、市場の可能性、研究開発への投資、新しい市場への取り組み、生産拠点と施設、会社の強みと弱み、製品の発売、製品試験パイプライン、製品の承認、特許、製品の幅と幅、アプリケーションの優位性、技術ライフライン曲線が含まれます。提供されている上記のデータ ポイントは、中東およびアフリカの遺伝子検査市場への会社の重点にのみ関連しています。
中東およびアフリカの遺伝子検査市場で活動している主要企業には、Abbott、23ANDME、Inc.、Danaher、Myriad Genetics、Inc.、OCP Medical Centre、LLC、Healthchecks360、Bayer AG、BioReference Health、LLC(OPKO Health、Inc.の子会社)、FREIBURG MEDICAL LABORATORY MIDDLE EAST(LLC)、Thermo Fisher Scientific Inc.、Bio-Rad Laboratories、Inc.、Biocartis、Eurofins Scientific、PerkinElmer Inc.、ELITechGroup、F. Hoffmann-La Roche Ltd.、Illumina、Inc.、QIAGEN、BIO-HELIX、PacBioなどがあります。
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。市場データは、市場統計モデルとコヒーレント モデルを使用して分析および推定されます。さらに、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数の市場への影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。これとは別に、データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、企業市場シェア分析、測定基準、中東およびアフリカと地域、ベンダー シェア分析が含まれます。さらに問い合わせる場合は、アナリストへの電話をリクエストしてください。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE MIDDLE EAST AND AFRICA GENETIC TESTING MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 YEARS CONSIDERED FOR THE STUDY
2.3 CURRENCY AND PRICING
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 MULTIVARIATE MODELLING
2.6 TYPE LIFELINE CURVE
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 MARKET APPLICATION COVERAGE GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL’S MODEL
4.2 PORTER'S FIVE FORCES MODEL
4.3 STRATEGIC INITIATIVES:
5 INDUSTRY INSIGHTS
5.1 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
5.2 CANCER GENETICS RISK ASSESSMENT AND COUNSELING
5.3 GENETIC TESTS PRICING AND KEY PRICING STRATEGIES
5.3.1 LAUNCH OF NEW TECHNOLOGY GENETIC TESTING KITS
5.3.2 PARTNERSHIPS WITH MARKET PLAYERS
5.3.3 PRENATAL PRICING STRATEGY
5.4 KEY PATIENT ENROLLMENT STRATEGIES
5.4.1 AWARENESS OF THE PUBLIC TOWARDS GENETIC TESTING TECHNOLOGY
5.4.2 GENETIC COUNSELLORS' SCOPE OF PRACTICE
5.4.3 EDUCATION AND COMMUNICATION
6 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: REGULATIONS
7 EPIDEMIOLOGY
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 GROWING PREVALENCE OF GENETIC DISORDERS
8.1.2 INCREASE IN THE ADOPTION OF NEXT GENERATION SEQUENCING
8.1.3 WIDE PRODUCT PORTFOLIO OFFERED BY MAJOR PLAYER
8.1.4 INCREASE TREND TOWARD PERSONALIZED MEDICATION
8.2 RESTRAINT
8.2.1 HIGH COST OF GENETIC TESTING
8.2.2 CYBER SECURITY CONCERN IN GENOMICS
8.3 OPPORTUNITIES
8.3.1 STRATEGIC INITIATIVES BY THE KEY MARKET PLAYER
8.3.2 TECHNOLOGICAL ADVANCEMENTS IN GENETIC TESTING
8.3.3 INCREASING RESEARCH AND DEVELOPMENT
8.3.4 RISING DISPOSABLE INCOME
8.4 CHALLENGES
8.4.1 LACK OF SKILLED PROFESSIONALS TO PERFORM GENETIC TESTING
8.4.2 STRINGENT REGULATION POLICY
9 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TYPE
9.1 OVERVIEW
9.2 DIAGNOSTIC TESTING
9.3 PRENATAL TESTING
9.3.1 NON-INVASIVE SCREENING
9.3.1.1 BY SCREENING METHOD
9.3.1.1.1 WHOLE GENOME SEQUENCING
9.3.1.1.2 COUNTING OF CFDNA FRAGMENTS
9.3.1.1.3 OTHERS
9.3.1.2 BY CONDITION
9.3.1.2.1 TRISOMY 21
9.3.1.2.2 KLINEFELTER SYNDROME
9.3.1.2.3 JACOBS SYNDROME
9.3.1.2.4 CYSTIC FIBROSIS
9.3.1.2.5 TURNER SYNDROME
9.3.1.2.6 TRISOMY 18
9.3.1.2.7 HEMOPHILIA
9.3.1.2.8 TRISOMY 13
9.3.1.2.9 MICRODELETION SYNDROME
9.3.1.2.10 FETAL GENDER
9.3.1.2.11 OTHERS
9.3.1.3 BY SCREENING TYPE
9.3.1.3.1 CARRIER SEQUENCING
9.3.1.3.2 SEQUENTIAL SEQUENCING
9.3.2 MATERNAL SERUM QUAD SCREENING
9.4 NEW BORN SCREENING
9.4.1 SICKLE CELL DISEASE
9.4.2 CONGENITAL HYPOTHYROIDISM
9.4.3 PHENYLKETONURINA (PKU)
9.4.4 GALACTOSEMIA
9.4.5 MAPLE SYRUP URINE DISEASE
9.4.6 OTHERS
9.5 PREDICTIVE AND PRESYMPTOMATIC TESTING
9.6 CARRIER TESTING
9.6.1 BY TEST TYPE
9.6.1.1 MOLECULAR SCREENING TEST
9.6.1.2 BIOCHEMICAL SCREENING TEST
9.6.2 BY TYPE
9.6.2.1 EXPANDED CARRIER SCREENING
9.6.2.1.1 PREDESIGNED PANEL TESTING
9.6.2.1.2 CUSTOM-MADE PANEL TESTING
9.6.2.2 TARGETED DISEASE CARRIER SCREENING
9.6.3 BY MEDICAL CONDITION
9.6.3.1 HEMATOLOGICAL CONDITIONS
9.6.3.2 PULMONARY CONDITIONS
9.6.3.3 NEUROLOGICAL CONDITIONS
9.6.3.4 OTHER CONDITIONS
9.7 OTHER TYPES
10 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TECHNOLOGY
10.1 OVERVIEW
10.2 POLYMERASE CHAIN REACTION
10.2.1 REAL-TIME PCR (QPCR)
10.2.2 DIGITAL PCR (DPCR)
10.2.3 REVERSE TRANSCRIPTION PCR (RT-PCR)
10.2.4 HOT-START PCR
10.2.5 MULTIPLEX PCR
10.2.6 OTHER PCR
10.3 DNA SEQUENCING (NGS-BASED TESTING)
10.3.1 NEXT GENERATION SEQUENCING (NGS)
10.3.2 SANGER SEQUENCING (SINGLE GENE)
10.3.3 OTHER
10.4 WHOLE GENOME SEQUENCING
10.5 MICROARRAYS
10.5.1 DNA MICROARRAYS
10.5.2 PROTEIN MICROARRAYS
10.5.3 OTHER MICROARRAYS
10.6 FLUORESCENCE IN SITU HYBRIDIZATION (FISH)
10.7 OTHERS
11 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY DISEASES
11.1 OVERVIEW
11.2 CANCER
11.2.1 BREAST
11.2.2 COLON
11.2.3 LUNG
11.2.4 PROSTATE
11.2.5 OTHERS
11.3 REPRODUCTIVE GENETIC TESTING
11.4 HEALTH AND WELLNESS GENETIC TESTING
11.5 SICKLE CELL ANEMIA
11.6 THALASSEMIA
11.7 RARE GENETIC DISORDER
11.7.1 TRISOMY 21
11.7.2 MONOSOMY X
11.7.3 TRISOMY 13
11.7.4 MICRODELETION SYNDROME
11.7.5 TRISOMY 18
11.7.6 OTHERS
11.8 FRAGILE X SYNDROME
11.9 DUCHENNE MUSCULAR DYSTROPHY
11.1 HUNTINGTON'S DISEASE
11.11 CYSTIC FIBROSIS
11.12 OTHERS
12 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS
12.3 CLINICS
12.4 DIAGNOSTIC CENTERS
12.5 PRIVATE CLINICS
12.6 LABORATORY SERVICE PROVIDERS
12.7 PRIVATE LABORATORIES
13 SUMMARY WRITE-UP (MIDDLE EAST AND AFRICA)
13.1 OVERVIEW
13.2 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY COUNTRY
13.2.1 SOUTH AFRICA
13.2.2 SAUDI ARABIA
13.2.3 U.A.E.
13.2.4 ISRAEL
13.2.5 EGYPT
13.2.6 REST OF MIDDLE EAST AND AFRICA
14 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: MIDDLE EAST AND AFRICA
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 BAYER AG
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 PRODUCT PORTFOLIO
16.1.4 RECENT DEVELOPMENTS
16.2 ABBOTT
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 PRODUCT PORTFOLIO
16.2.4 RECENT DEVELOPMENTS
16.3 ILLUMINA, INC.
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 PRODUCT PORTFOLIO
16.3.4 RECENT DEVELOPMENTS
16.4 THERMO FISHER SCIENTIFIC INC.
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 PRODUCT PORTFOLIO
16.4.4 RECENT DEVELOPMENTS
16.5 F.HOFFAMANN-LA ROCHE LTD.
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 PRODUCT PORTFOLIO
16.5.4 RECENT DEVELOPMENTS
16.6 BIOREFERNCE HEALTH INC. (SUBSIDIARY OPKO HEALTH, INC.)
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENTS
16.7 ELITECHGROUP
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENTS
16.8 23ANDME, INC.
16.8.1 COMPANY SNAPSHOT
16.8.2 REVENUE ANALYSIS
16.8.3 PRODUCT PORTFOLIO
16.8.4 RECENT DEVELOPMENTS
16.9 BIOCARTIS
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT DEVELOPMENTS
16.1 BIO-HELIX
16.10.1 COMPANY SNAPSHOT
16.10.2 PRODUCT PORTFOLIO
16.10.3 RECENT DEVELOPMENTS
16.11 BIO-RAD LABORATORIES, INC.
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUE ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT DEVELOPMENTS
16.12 DANAHER
16.12.1 COMPANY SNAPSHOT
16.12.2 REVENUE ANALYSIS
16.12.3 PRODUCT PORTFOLIO
16.12.4 RECENT DEVELOPMENTS
16.13 EUROFINS SCIENTIFIC
16.13.1 COMPANY SNAPSHOT
16.13.2 REVENUE ANALYSIS
16.13.3 PRODUCT PORTFOLIO
16.13.4 RECENT DEVELOPMENTS
16.14 FREIBURG MEDICAL LABORATORY MIDDLE EAST (L.L.C)
16.14.1 COMPANY SNAPSHOT
16.14.2 PRODUCT PORTFOLIO
16.14.3 RECENT DEVELOPMENTS
16.15 HEALTHCHECKS360
16.15.1 COMPANY SNAPSHOT
16.15.2 PRODUCT PORTFOLIO
16.15.3 RECENT DEVELOPMENTS
16.16 MYRIAD GENETICS, INC.
16.16.1 COMPANY SNAPSHOT
16.16.2 REVENUE ANALYSIS
16.16.3 PRODUCT PORTFOLIO
16.16.4 RECENT DEVELOPMENTS
16.17 OCP MEDICAL CENTER L.L.C
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENTS
16.18 PACBIO
16.18.1 COMPANY SNAPSHOT
16.18.2 REVENUE ANALYSIS
16.18.3 PRODUCT PORTFOLIO
16.18.4 RECENT DEVELOPMENTS
16.19 PERKINELMER ONC.
16.19.1 COMPANY SNAPSHOT
16.19.2 REVENUE ANALYSIS
16.19.3 PRODUCT PORTFOLIO
16.19.4 RECENT DEVELOPMENTS
16.2 QIAGEN
16.20.1 COMPANY SNAPSHOT
16.20.2 REVENUE ANALYSIS
16.20.3 PRODUCT PORTFOLIO
16.20.4 RECENT DEVELOPMENTS
17 QUESTIONNAIRE
18 RELATED REPORTS
表のリスト
TABLE 1 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 2 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 3 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 4 MIDDLE EAST AND AFRICA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 5 MIDDLE EAST AND AFRICA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 6 MIDDLE EAST AND AFRICA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 7 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (USD MILLION)
TABLE 8 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (UNITS)
TABLE 9 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (ASP)
TABLE 10 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2021-2030 (USD MILLION)
TABLE 11 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (USD MILLION)
TABLE 12 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (UNITS)
TABLE 13 MIDDLE EAST AND AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (ASP)
TABLE 14 MIDDLE EAST AND AFRICA NEW BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 15 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 16 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 17 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 18 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 19 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 20 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 21 MIDDLE EAST AND AFRICA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 22 MIDDLE EAST AND AFRICA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 23 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 24 MIDDLE EAST AND AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION 2021-2030 (USD MILLION)
TABLE 25 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 26 MIDDLE EAST AND AFRICA POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 27 MIDDLE EAST AND AFRICA DNA SEQUENCING (NGS-BASED TESTING) IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 28 MIDDLE EAST AND AFRICA MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 29 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET , BY DISEASES, 2021-2030 (USD MILLION)
TABLE 30 MIDDLE EAST AND AFRICA CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 31 MIDDLE EAST AND AFRICA RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 32 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 33 IDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY COUNTRY 2021-2030 (USD MILLION)
TABLE 34 SOUTH AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 35 SOUTH AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 36 SOUTH AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 37 SOUTH AFRICA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 38 SOUTH AFRICA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 39 SOUTH AFRICA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 40 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (USD MILLION)
TABLE 41 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (UNITS)
TABLE 42 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (ASP)
TABLE 43 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2021-2030 (USD MILLION)
TABLE 44 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (USD MILLION)
TABLE 45 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (UNITS)
TABLE 46 SOUTH AFRICA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (ASP)
TABLE 47 SOUTH AFRICA NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 48 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 49 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 50 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (ASP)
TABLE 51 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 52 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 53 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 54 SOUTH AFRICA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 55 SOUTH AFRICA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 56 SOUTH AFRICA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 57 SOUTH AFRICA CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2021-2030 (USD MILLION)
TABLE 58 SOUTH AFRICA GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 59 SOUTH AFRICA POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 60 SOUTH AFRICA DNA SEQUENCING (NGS-BASED TESTING) IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 61 SOUTH AFRICA MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 62 SOUTH AFRICA GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 63 SOUTH AFRICA RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 64 SOUTH AFRICA CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 65 SOUTH AFRICA GENETIC TESTING MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 66 SAUDI ARABIA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 67 SAUDI ARABIA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 68 SAUDI ARABIA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 69 SAUDI ARABIA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 70 SAUDI ARABIA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 71 SAUDI ARABIA PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 72 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (USD MILLION)
TABLE 73 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (UNITS)
TABLE 74 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (ASP)
TABLE 75 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2021-2030 (USD MILLION)
TABLE 76 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (USD MILLION)
TABLE 77 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (UNITS)
TABLE 78 SAUDI ARABIA NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (ASP)
TABLE 79 SAUDI ARABIA NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 80 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 81 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 82 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (ASP)
TABLE 83 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 84 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 85 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 86 SAUDI ARABIA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 87 SAUDI ARABIA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 88 SAUDI ARABIA EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 89 SAUDI ARABIA CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2021-2030 (USD MILLION)
TABLE 90 SAUDI ARABIA GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 91 SAUDI ARABIA POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 92 SAUDI ARABIA DNA SEQUENCING (NGS-BASED TESTING) IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 93 SAUDI ARABIA MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 94 SAUDI ARABIA GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 95 SAUDI ARABIA RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 96 SAUDI ARABIA CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 97 SAUDI ARABIA GENETIC TESTING MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 98 U.A.E. GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 99 U.A.E. GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 100 U.A.E. GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 101 U.A.E. PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 102 U.A.E. PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 103 U.A.E. PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 104 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (USD MILLION)
TABLE 105 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (UNITS)
TABLE 106 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (ASP)
TABLE 107 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2021-2030 (USD MILLION)
TABLE 108 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (USD MILLION)
TABLE 109 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (UNITS)
TABLE 110 U.A.E. NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (ASP)
TABLE 111 U.A.E. NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 112 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 113 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 114 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (ASP)
TABLE 115 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 116 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 117 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 118 U.A.E. EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 119 U.A.E. EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 120 U.A.E. EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 121 U.A.E. CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2021-2030 (USD MILLION)
TABLE 122 U.A.E. GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 123 U.A.E. POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 124 U.A.E. DNA SEQUENCING (NGS-BASED TESTING) IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 125 U.A.E. MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 126 U.A.E. GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 127 U.A.E. RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 128 U.A.E. CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 129 U.A.E. GENETIC TESTING MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 130 ISRAEL GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 131 ISRAEL GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 132 ISRAEL GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 133 ISRAEL PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 134 ISRAEL PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 135 ISRAEL PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 136 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (USD MILLION)
TABLE 137 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (UNITS)
TABLE 138 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (ASP)
TABLE 139 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2021-2030 (USD MILLION)
TABLE 140 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (USD MILLION)
TABLE 141 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (UNITS)
TABLE 142 ISRAEL NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (ASP)
TABLE 143 ISRAEL NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 144 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 145 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 146 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (ASP)
TABLE 147 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 148 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 149 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 150 ISRAEL EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 151 ISRAEL EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 152 ISRAEL EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 153 ISRAEL CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2021-2030 (USD MILLION)
TABLE 154 ISRAEL GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 155 ISRAEL POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 156 ISRAEL DNA SEQUENCING (NGS-BASED TESTING) IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 157 ISRAEL MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 158 ISRAEL GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 159 ISRAEL RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 160 ISRAEL CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 161 ISRAEL GENETIC TESTING MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 162 EGYPT GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 163 EGYPT GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 164 EGYPT GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 165 EGYPT PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 166 EGYPT PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 167 EGYPT PRENATAL TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 168 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (USD MILLION)
TABLE 169 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (UNITS)
TABLE 170 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING METHOD, 2021-2030 (ASP)
TABLE 171 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY CONDITION, 2021-2030 (USD MILLION)
TABLE 172 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (USD MILLION)
TABLE 173 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (UNITS)
TABLE 174 EGYPT NON-INVASIVE SCREENING IN GENETIC TESTING MARKET, BY SCREENING TYPE, 2021-2030 (ASP)
TABLE 175 EGYPT NEW-BORN SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 176 EGYPT CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 177 EGYPT CARRIER TESTING IN GENETIC TESTING MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 178 EGYPT CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 179 EGYPT CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 180 EGYPT CARRIER TESTING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 181 EGYPT EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 182 EGYPT EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (UNITS)
TABLE 183 EGYPT EXPANDED CARRIER SCREENING IN GENETIC TESTING MARKET, BY TYPE, 2021-2030 (ASP)
TABLE 184 EGYPT CARRIER TESTING IN GENETIC TESTING MARKET, BY MEDICAL CONDITION, 2021-2030 (USD MILLION)
TABLE 185 EGYPT GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 186 EGYPT POLYMERASE CHAIN REACTION IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 187 EGYPT DNA SEQUENCING (NGS-BASED TESTING) IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 188 EGYPT MICROARRAYS IN GENETIC TESTING MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 189 EGYPT GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 190 EGYPT RARE GENETIC DISORDER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 191 EGYPT CANCER IN GENETIC TESTING MARKET, BY DISEASES, 2021-2030 (USD MILLION)
TABLE 192 EGYPT GENETIC TESTING MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 193 REST OF MIDDLE EAST AND AFRICA GENETIC TESTING MARKET, BY TYPE, 2021-2030 (USD MILLION)
図表一覧
FIGURE 1 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET:DATA TRIANGULATION
FIGURE 3 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET :COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET : INTERVIEW DEMOGRAPHICS
FIGURE 7 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET : DBMR MARKET POSITION GRID
FIGURE 8 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET : MARKET APPLICATION COVERAGE GRID
FIGURE 9 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: SEGMENTATION
FIGURE 11 THE INCREASING PREVALENCE OF GENETIC DISEASES AND RISING HEALTHCARE EXPENDITURE ARE EXPECTED TO DRIVE THE MIDDLE EAST AND AFRICA GENETIC TESTING MARKET GROWTH IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 12 DIGANOSTIC TESTING SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST AND AFRICA GENETIC TESTING MARKET IN 2023 & 2030
FIGURE 13 EPIDEMIOLOGY
FIGURE 14 INCIDENCE OF ALL GENDER-SOUTH AFRICA AND SAUDI ARABIA
FIGURE 15 INCIDENCE OF ALL GENDER-UAE AND EGYPT
FIGURE 16 INCIDENCE OF ALL GENDER-ISRAEL
FIGURE 17 MORTALITY RATE- SOUTH AFRICA AND SAUDI ARABIA
FIGURE 18 MORTALITY RATE-UAE AND EGYPT
FIGURE 19 MORTALITY RATE-ISRAEL
FIGURE 20 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE MIDDLE EAST AND AFRICA GENETIC TESTING MARKET
FIGURE 21 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TYPE, 2022
FIGURE 22 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 23 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 24 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TYPE, LIFELINE CURVE
FIGURE 25 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TECHNOLOGY, 2022
FIGURE 26 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TECHNOLOGY, 2023-2030 (USD MILLION)
FIGURE 27 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TECHNOLOGY, CAGR (2023-2030)
FIGURE 28 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TECHNOLOGY, LIFELINE CURVE
FIGURE 29 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY DISEASES, 2022
FIGURE 30 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY DISEASES, 2023-2030 (USD MILLION)
FIGURE 31 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY DISEASES, CAGR (2023-2030)
FIGURE 32 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY DISEASES, LIFELINE CURVE
FIGURE 33 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY END USER, 2022
FIGURE 34 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 35 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY END USER, CAGR (2023-2030)
FIGURE 36 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY END USER, LIFELINE CURVE
FIGURE 37 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: SNAPSHOT (2022)
FIGURE 38 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY COUNTRY (2022)
FIGURE 39 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY COUNTRY (2023 & 2030)
FIGURE 40 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY COUNTRY (2022 & 2030)
FIGURE 41 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: BY TYPE (2023-2030)
FIGURE 42 MIDDLE EAST AND AFRICA GENETIC TESTING MARKET: COMPANY SHARE 2022 (%)

調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。













