Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market to 2032
Overview
The Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market is expected to reach a 4,730.84 USD Billion by 2032 and is projected to grow at a CAGR of 22.69% from 2025 to 2032.
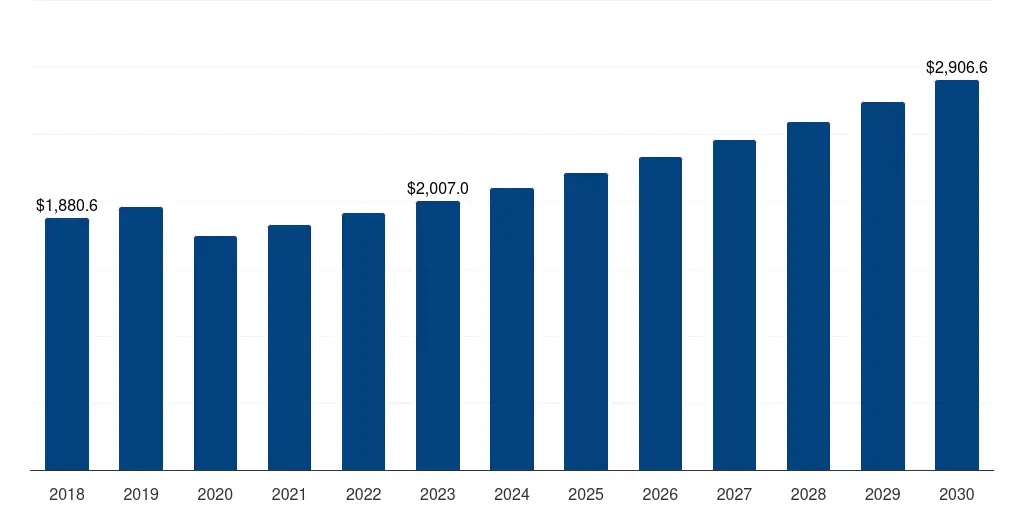
Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market 2018-2032 USD Billion
Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market, Key Findings (2025-2032)
Market Growth and Projections:
- Market Size (2024): 1,649.32 USD Billion
- Projected Market Size (2032): 4,730.84 USD Billion
- CAGR (2025-2032): 22.69%
Key Findings of Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market
- The Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market was valued at 1,649.32 USD Billion in 2024.
- The Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market is likely to grow at a CAGR of 22.69% during the forecast period of 2024 to 2032.
- In 2024, the Largest segment Regulatory Affairs Services in Services Segment accounted for the largest share of the market with a revenue of 1,053.67 USD Billion
- The fastest growing segment Cardiology in Application Segment grew Fastest with a CAGR of 31.11% during the forecast period from 2024 to 2032.
Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market Scope
- Drug Delivery
- Diabetes Care
- Endoscopy
- Opthalmic
- Dental
- Diagnostic Imaging
- Orthopedic
- IVD
- Others
- General and Plastic Surgery
- Cardiology
- Class III
- Class II
- Class I
- Medical Writing
- Quality Consulting
- Regulatory Affairs Services
- Raw Materials
- Electronics
- Finished Goods
- Large Medical Device Company
- Small Medical Device Company
- Medium Medical Device Company
Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market Data Coverage Insights
| Study Period | 2024-2032 |
| Base Year | 2020 |
| Unit | Revenue in USD Billion |
| Market Value in 2024 | 1,649.32 USD Billion |
| Market Value in 2032 | 4,730.84 USD Billion |
| CAGR (2025-2032) | 22.69% |
| Historic Data | 2016-2023 |
| Market Segments Covered | Application,Device Type,Services,Product,End User |
Regional Insights:
-
Leading Market (2024-2032): Asia-Pacific, leading in terms of revenue 1,649.32 USD Billion in 2024
- Key Country: China, leading in terms of revenue with value of 396.90 USD Billion in 2024.
Segments and Scope
-
Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market to 2032, By Application
- Cardiology is the largest segment in Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market to 2032 with a revenue of 381.79 USD Billion in the year 2024.
- Cardiology is the Fastest growing segment in Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market to 2032 with a Growth rate of 31.11 % in forecast period 2025-2032.
-
Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market to 2032, By Device Type
- Class I is the largest segment in Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market to 2032 with a revenue of 737.24 USD Billion in the year 2024.
- Class I is the Fastest growing segment in Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market to 2032 with a Growth rate of 26.88 % in forecast period 2025-2032.
-
Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market to 2032, By Services
- Regulatory Affairs Services is the largest segment in Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market to 2032 with a revenue of 1,053.67 USD Billion in the year 2024.
- Regulatory Affairs Services is the Fastest growing segment in Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market to 2032 with a Growth rate of 24.16 % in forecast period 2025-2032.
-
Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market to 2032, By Product
- Finished Goods is the largest segment in Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market to 2032 with a revenue of 796.87 USD Billion in the year 2024.
- Finished Goods is the Fastest growing segment in Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market to 2032 with a Growth rate of 26.36 % in forecast period 2025-2032.
-
Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market to 2032, By End User
- Medium Medical Device Company is the largest segment in Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market to 2032 with a revenue of 813.88 USD Billion in the year 2024.
- Medium Medical Device Company is the Fastest growing segment in Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market to 2032 with a Growth rate of 26.14 % in forecast period 2025-2032.
Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market Company Share Analysis
| Company Name |

|
||
| Parexel International Corporation | |||
| North American Science Associates, LLC | |||
| Integer Holdings Corporation | |||
| Labcorp Drug Development | |||
| TÜV SÜD | |||

Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market Geographical Sales Distribution, 2018-2032 USD Billion
Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market Company Profiling

Industry Related Reports
Frequently Asked Questions
Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market Scope
- Drug Delivery
- Diabetes Care
- Endoscopy
- Opthalmic
- Dental
- Diagnostic Imaging
- Orthopedic
- IVD
- Others
- General and Plastic Surgery
- Cardiology
- Class III
- Class II
- Class I
- Medical Writing
- Quality Consulting
- Regulatory Affairs Services
- Raw Materials
- Electronics
- Finished Goods
- Large Medical Device Company
- Small Medical Device Company
- Medium Medical Device Company
Frequently Asked Questions
Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market Company Profiling

Frequently Asked Questions
CHOOSE LICENCE TYPE
- 4200.00
- 3500.00
- 2000.00
- 5500.00
- 8500.00
HAVE A QUESTION?
Get expert advice on the right strategy for your business at no cost. We also offer customized subscription plans and affordable discounts for startups and universities.