Global Auto Disable Syringes Market
Market Size in USD Billion
CAGR :
% 
 USD
29.39 Billion
USD
72.09 Billion
2025
2033
USD
29.39 Billion
USD
72.09 Billion
2025
2033
| 2026 –2033 | |
| USD 29.39 Billion | |
| USD 72.09 Billion | |
|
|
|
|
Auto Disable Syringes Market Size
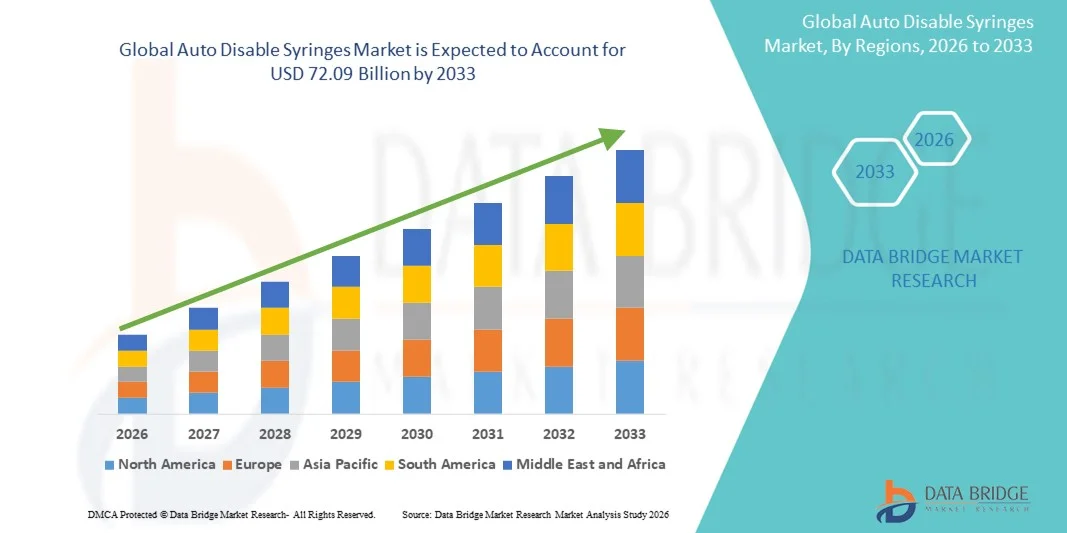
- The global auto disable syringes market size was valued at USD 29.39 billion in 2025 and is expected to reach USD 72.09 billion by 2033, at a CAGR of 11.87% during the forecast period
- The market growth is largely fueled by the rising emphasis on safe injection practices and continuous technological advancements in syringe design, leading to increased adoption of auto disable syringes across hospitals, vaccination programs, and public health initiatives in both urban and rural settings
- Furthermore, growing awareness of needlestick injury prevention, the global push to eliminate syringe reuse, and increasing government mandates for single-use injection devices are establishing auto disable syringes as the preferred standard for immunization and therapeutic procedures. These converging factors are accelerating the uptake of Auto Disable Syringes solutions, thereby significantly boosting the industry’s growth
Auto Disable Syringes Market Analysis
- Auto Disable Syringes, designed for single-use to prevent reuse and cross-contamination, are critical tools in ensuring safe injection practices across hospitals, vaccination programs, and public health campaigns due to their built-in safety mechanisms that automatically lock after use
- The rising demand for auto disable syringes is primarily driven by increasing immunization initiatives, growing awareness of needlestick injury prevention, strict government regulations on safe injection practices, and the expansion of global vaccination programs
- North America dominated the auto disable syringes market with the largest revenue share of 38.6% in 2025, supported by advanced healthcare infrastructure, strong government regulations on injection safety, high vaccination rates, and widespread adoption of safety-engineered medical devices in hospitals and clinics
- Asia-Pacific is expected to be the fastest growing region in the auto disable syringes market during the forecast period, fueled by large-scale immunization campaigns, rapidly expanding healthcare infrastructure, increasing population, and rising government investments in public health and disease prevention
- The vaccines segment accounted for the largest market revenue share of roughly 52.3% in 2025, as auto-disable syringes are mandatory in most national immunization programs to avoid syringe reuse and prevent blood-borne infections
Report Scope and Auto Disable Syringes Market Segmentation
|
Attributes |
Auto Disable Syringes Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
• Terumo Corporation (Japan) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Auto Disable Syringes Market Trends
Advancements in Safety Mechanisms and Infection Control
- A significant and accelerating trend in the global Auto Disable Syringes market is the increasing focus on enhanced safety mechanisms and stricter infection prevention standards across healthcare systems. This growing emphasis on patient and healthcare worker safety is driving widespread adoption of auto disable syringes in immunization programs, therapeutic injections, and emergency care settings. These devices are designed to automatically lock or break after a single use, effectively preventing reuse and reducing the risk of transmission of blood-borne diseases such as HIV, hepatitis B, and hepatitis C
- For instance, several international vaccination campaigns supported by global health organizations have increasingly mandated the use of auto disable syringes to ensure safe injection practices. As a result, manufacturers are developing advanced single-use syringe designs with improved plunger mechanisms and tamper-proof features to comply with international safety guidelines. These innovations are particularly important in mass immunization programs and pediatric vaccination drives
- The integration of improved engineering designs has enabled auto disable syringes to offer greater reliability, consistent dosage delivery, and smoother administration. Enhanced needle technology, reduced dead space, and ergonomically designed plungers contribute to better clinical outcomes while minimizing medication wastage. These design improvements also support higher efficiency in high-volume healthcare settings such as vaccination centers, hospitals, and mobile clinics
- The growing involvement of government bodies, non-governmental organizations, and international agencies in promoting safe injection practices is further accelerating market demand. Large-scale procurement programs for national immunization initiatives rely heavily on auto disable syringes as a standard option, creating sustained demand across both developed and developing regions. This centralized distribution approach supports wide-scale adoption in regions with limited access to advanced healthcare infrastructure
- This trend toward safer, standardized, and tamper-proof injection devices is fundamentally reshaping expectations in the medical device industry. Consequently, manufacturers are investing in research and development to improve cost-efficiency, scalability, and user-friendliness of auto disable syringe products, ensuring compliance with evolving global healthcare standards and regulations
- The demand for auto disable syringes is growing rapidly across public health organizations, hospitals, clinics, and humanitarian aid programs, as stakeholders increasingly prioritize infection control, safety compliance, and reliable performance in both routine and emergency healthcare operations
Auto Disable Syringes Market Dynamics
Driver
Growing Demand Due to Expanding Immunization Programs and Rising Awareness of Safe Injection Practices
- The rapid expansion of immunization programs worldwide, combined with increasing awareness of safe injection practices, is a major driver of growth in the auto disable syringes market. Governments and global health agencies are continuously scaling up vaccination coverage to prevent the spread of infectious diseases, which significantly increases the demand for safe, single-use injection devices
- For instance, in April 2025, a leading medical device manufacturer announced expanded production capacity for safety syringes to support large-scale vaccination initiatives in developing regions. Such strategic developments by key industry players are expected to contribute to the sustained growth of the auto disable syringes market during the forecast period
- As healthcare providers become more aware of the dangers associated with needle reuse and improper disposal, there is a stronger shift toward the adoption of safety-engineered injection devices. Auto disable syringes offer an effective solution by ensuring that each syringe can only be used once, reducing the risk of cross-contamination and improving overall patient safety
- Furthermore, the rising prevalence of chronic diseases such as diabetes, cancer, and cardiovascular conditions has increased the need for frequent injectable drug administration. This has led to higher consumption of syringes in both hospital and home-care settings, further reinforcing the demand for auto disable variants that offer added safety benefits
- The increasing presence of healthcare infrastructure improvements in low- and middle-income countries, supported by international funding and development programs, is also strengthening market expansion. These investments ensure better access to safe medical devices and promote adherence to global health and safety protocols
- The combination of growing vaccination coverage, increased healthcare spending, and strong regulatory support for single-use devices continues to create a favorable environment for the long-term growth of the auto disable syringes market
Restraint/Challenge
Cost Constraints, Supply Chain Limitations, and Environmental Concerns
- Despite the strong benefits, the relatively higher cost of auto disable syringes compared to conventional disposable syringes remains a notable challenge, particularly in resource-limited settings. Budget constraints in public healthcare systems may limit large-scale procurement, especially in rural or underdeveloped regions where funding is already stretched
- For instance, in some low-income countries, healthcare facilities are forced to prioritize lower-cost medical supplies, which can slow the transition from standard disposable syringes to more advanced auto disable alternatives. This can result in uneven adoption rates and continued reliance on less secure injection practices in certain areas
- In addition, supply chain disruptions, raw material shortages, and logistical challenges can impact the consistent availability of auto disable syringes. Global events such as pandemics or geopolitical tensions may affect manufacturing and distribution networks, leading to delays in delivery and increased operational costs for healthcare providers and governments
- The environmental impact of single-use plastic medical products also presents a growing concern. Large-scale use of auto disable syringes leads to increased medical waste, and improper disposal can contribute to environmental pollution. Managing and treating this waste requires effective infrastructure, which is not always available in developing countries
- While technological advancements continue to improve production efficiency, price sensitivity remains a key issue in many regions. Healthcare providers may hesitate to invest in higher-priced safety syringes without clear reimbursement policies or government support
- Overcoming these barriers through sustainable material innovations, improved waste-management practices, enhanced local manufacturing capabilities, and stronger financial support mechanisms will be essential to ensure long-term, equitable growth of the global auto disable syringes market
Auto Disable Syringes Market Scope
The market is segmented on the basis of mechanism, clinical indication, application, and end user.
- By Mechanism
On the basis of mechanism, the Global Auto Disable Syringes market is segmented into retractable, non-retractable, and others. The non-retractable segment dominated the largest market revenue share of approximately 45.6% in 2025, primarily due to its widespread availability, low manufacturing cost, and extensive use in large-scale immunization programs across developing and developed regions. These syringes are designed to lock or break after a single use, preventing reuse and cross-contamination. Governments and public health agencies continue to prioritize non-retractable auto-disable syringes in vaccination campaigns for diseases such as measles, polio, COVID-19, and hepatitis due to their operational simplicity. Their strong adoption in resource-limited healthcare settings further strengthens the segment's dominance, as they do not require complex training for safe handling. In addition, the availability of these syringes in a wide range of sizes enhances their adaptability for different injections. Continuous procurement by global health organizations also contributes to steady demand, making this segment the backbone of the auto-disable syringes market globally.
The retractable segment is anticipated to witness the fastest CAGR of around 11.8% from 2026 to 2033, driven by rising focus on healthcare worker safety and strict regulations related to needlestick injuries. Retractable syringes feature an automatic needle retraction mechanism post-injection, significantly reducing the risk of accidental needle pricks, which are a major concern in hospitals and clinics. Growing awareness of occupational hazards among medical professionals is accelerating the preference for these advanced safety syringes. In developed regions, particularly North America and Europe, healthcare institutions are rapidly shifting towards retractable technologies to improve workplace safety standards. Technological innovation in syringe design and an increase in funding for safer injection practices are also aiding market growth. In addition, hospitals are willingly adopting retractable syringes despite their higher cost due to long-term benefits such as reduced injury claims and compliance with safety regulations. As healthcare infrastructure modernizes globally, the demand for these advanced syringes is expected to rise substantially.
- By Clinical Indication
On the basis of clinical indication, the Global Auto Disable Syringes market is segmented into vaccines, infectious diseases, inflammatory/auto-immune diseases, and others. The vaccines segment accounted for the largest market revenue share of roughly 52.3% in 2025, as auto-disable syringes are mandatory in most national immunization programs to avoid syringe reuse and prevent blood-borne infections. Mass vaccination drives for childhood immunization, pandemic preparedness, and booster programs have significantly strengthened the demand for auto-disable syringes in this segment. Global initiatives by WHO, UNICEF, and GAVI have further increased procurement and distribution volumes, especially in emerging economies. The rising emphasis on eliminating diseases such as polio, measles, and tetanus also supports sustained demand. In addition, annual influenza vaccination programs and the expansion of adult vaccination campaigns are driving continuous consumption. Governments prefer auto-disable syringes in vaccination settings due to their built-in safety and tamper-proof nature. All these factors combine to make vaccines the most dominant clinical segment in the global market.
The infectious diseases segment is expected to register the fastest CAGR of around 10.6% from 2026 to 2033, due to the increasing prevalence of diseases such as HIV, tuberculosis, malaria, and hepatitis worldwide. Regular administration of injectable antibiotics, antivirals, and antimalarial medications is creating consistent demand for safe, single-use injection devices. Growing concerns about hospital-acquired infections and improper syringe disposal are pushing healthcare facilities to adopt auto-disable syringes as a safer alternative. In regions with high disease burden, governments are strengthening healthcare infrastructure and expanding access to treatment, which further accelerates growth. International aid programs and rising investments in infectious disease control are also boosting adoption. Moreover, the frequency of injections in long-term treatment regimens contributes to higher syringe consumption. Technological advancements making these syringes more affordable have further facilitated their penetration in low-income areas. As infectious diseases remain a global health challenge, this segment is expected to show strong and sustained growth.
- By Application
On the basis of application, the Global Auto Disable Syringes market is segmented into blood collection, vaccination, drug delivery, and others. The vaccination segment held the largest market revenue share of approximately 49.8% in 2025, driven by large-scale government immunization initiatives and global vaccination campaigns. Auto disable syringes are especially preferred in vaccination because they ensure one-time use and eliminate the possibility of reuse, which is critical in preventing disease transmission. The increasing frequency of vaccination programs for both children and adults generates large recurring demand. National health authorities mandate the use of safe injection devices for immunization, which further reinforces market dominance. The rise in awareness about safe injection practices among healthcare professionals and communities also supports segment growth. In addition, the continuous introduction of new vaccines for emerging diseases expands application scope. Seasonal vaccination programs such as flu and COVID boosters contribute consistently to high volume usage. As a result, vaccination remains the leading application area for auto-disable syringes worldwide.
The drug delivery segment is expected to grow at the fastest CAGR of around 12.1% from 2026 to 2033, supported by the growing prevalence of chronic diseases such as diabetes, cancer, and autoimmune disorders that require frequent injectable therapeutics. Patients undergoing long-term treatments benefit from the safety advantages of single-use syringes, reducing infection risk and enhancing treatment compliance. Hospitals and home healthcare services are increasingly adopting auto-disable syringes for therapeutic injections to ensure contamination-free drug administration. The expansion of biologic drugs and biosimilars that must be administered via injection is also contributing to fast growth in this segment. In addition, increasing self-administration trends in certain therapies are supporting demand for user-friendly and safe syringe designs. Pharmaceutical companies and healthcare providers are working together to promote safe drug delivery tools to improve treatment outcomes. As injectable medications become more common, the need for auto-disable syringes in drug delivery continues to strengthen. This sustained demand positions drug delivery as the fastest-growing application segment in the coming years.
- By End User
On the basis of end user, the Global Auto Disable Syringes market is segmented into hospitals, clinics, diagnostic laboratories, ambulatory surgical centers, and others. The hospitals segment dominated the largest market revenue share of around 47.4% in 2025, as hospitals are primary centers for immunization, emergency care, surgical procedures, and patient treatment. The high volume of injections administered daily in hospitals naturally drives increased consumption of auto-disable syringes. Hospitals follow strict infection control protocols, which makes the use of single-use, safety-enabled syringes mandatory in most departments. The presence of trained healthcare professionals and government supply agreements also ensures continuous procurement. In addition, the rising patient footfall due to population growth, aging demographics, and chronic disease prevalence is further pushing demand. Hospitals prefer auto-disable syringes due to their efficiency, reliability, and ability to minimize medical waste misuse. This strong infrastructure and large-scale usage make hospitals the leading end-user segment in the global market.
The ambulatory surgical centers (ASCs) segment is projected to witness the fastest CAGR of approximately 11.3% from 2026 to 2033, driven by the increasing shift towards outpatient procedures and minimally invasive surgeries. These facilities require safe, single-use syringes for anesthesia, medication administration, and post-operative treatments. As patients increasingly prefer outpatient settings due to lower costs and faster recovery times, the number of procedures performed in ASCs is rising rapidly. This is creating consistent demand for advanced safety injection devices. The growing establishment of new ambulatory care centers globally further boosts market opportunities. ASCs emphasize high standards of hygiene and infection prevention, which aligns perfectly with the features of auto-disable syringes. In addition, the faster turnaround of patients in these centers increases syringe consumption rates per day. This strong expansion trend is expected to make ASCs one of the most dynamic growth areas in the forecast period.
Auto Disable Syringes Market Regional Analysis
- North America dominated the auto disable syringes market with the largest revenue share of 38.6% in 2025, supported by advanced healthcare infrastructure, strong government regulations on injection safety, high vaccination rates, and the widespread adoption of safety-engineered medical devices in hospitals and clinics
- The presence of leading medical device manufacturers, coupled with strict OSHA and CDC guidelines to prevent needlestick injuries, has significantly increased the demand for auto disable syringes across the region
- In addition, well-established immunization programs for both pediatric and adult populations further contribute to consistent market growth. Increased awareness regarding hospital-acquired infections and the importance of safe injection practices among healthcare workers are also reinforcing the dominant position of North America in the global market
U.S. Auto Disable Syringes Market Insight
The U.S. auto disable syringes market captured the largest revenue share within North America in 2025, driven by stringent healthcare regulations, large-scale vaccination initiatives, and a strong focus on healthcare worker safety. Federal mandates requiring the use of safety syringes in hospitals and outpatient settings have encouraged rapid adoption of auto disable technologies. In addition, high healthcare spending, widespread insurance coverage, and continuous innovation in syringe safety designs are significantly strengthening market demand. The rising prevalence of chronic diseases requiring injectable treatments, alongside regular immunization programs, is further accelerating the use of auto disable syringes across hospitals, clinics, and public health organizations in the country.
Europe Auto Disable Syringes Market Insight
The Europe auto disable syringes market is projected to expand at a steady CAGR throughout the forecast period, primarily driven by strict regulatory frameworks related to healthcare safety and infection control. European countries place strong emphasis on preventing cross-contamination and ensuring safe medical practices in both public and private healthcare settings. Increasing vaccination coverage, an aging population, and rising prevalence of chronic disorders are contributing to the growing demand for safe injection devices. Additionally, ongoing government initiatives to strengthen healthcare systems and modernize medical infrastructure are supporting the wider adoption of auto disable syringes across hospitals, diagnostic laboratories, and vaccination centers throughout the region.
U.K. Auto Disable Syringes Market Insight
The U.K. auto disable syringes market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by the National Health Service’s (NHS) continuous efforts to improve patient safety and reduce medical errors. The country’s strong focus on minimizing needlestick injuries among healthcare professionals has increased demand for safety-engineered syringe systems. In addition, public health campaigns promoting vaccination and disease prevention, coupled with increasing outpatient treatments, are boosting the consumption of auto disable syringes. The rise in nursing homes, community clinics, and home healthcare services is also contributing to market growth in the U.K.
Germany Auto Disable Syringes Market Insight
The Germany auto disable syringes market is expected to expand at a considerable CAGR during the forecast period, fueled by the country’s highly developed healthcare system, strong emphasis on medical safety, and technological advancement in medical devices. Germany’s extensive network of hospitals and specialized clinics creates a high and consistent demand for safe injection systems. The country’s leadership in pharmaceutical production and clinical research also requires the use of safe, single-use injection tools. Additionally, rising awareness regarding medical waste management and infection prevention is driving healthcare facilities to shift rapidly towards auto disable syringes as a standard practice.
Asia-Pacific Auto Disable Syringes Market Insight
The Asia-Pacific auto disable syringes market is expected to be the fastest growing region during the forecast period, driven by large-scale immunization campaigns, rapidly expanding healthcare infrastructure, increasing population, and rising government investments in public health and disease prevention. Countries across the region are actively strengthening their vaccination programs, particularly for children and vulnerable populations, which is significantly boosting the demand for auto disable syringes. Growing awareness regarding the dangers of syringe reuse and rising efforts to improve infection control standards are also supporting adoption. Furthermore, international support from organizations such as WHO and UNICEF is accelerating the supply of safety syringes in developing countries across the Asia-Pacific region.
Japan Auto Disable Syringes Market Insight
The Japan auto disable syringes market is gaining momentum due to the country’s aging population, advanced healthcare system, and strong commitment to patient and worker safety. The increased need for routine vaccinations, chronic disease management, and geriatric care is driving the consumption of single-use, safety-enabled syringes. Japan’s focus on adopting innovative medical technologies and maintaining strict hygiene standards in healthcare facilities is further supporting market expansion. The growing demand for convenient, reliable, and contamination-free injection methods in both hospitals and home care settings is positively influencing the market outlook.
China Auto Disable Syringes Market Insight
The China auto disable syringes market accounted for the largest market revenue share in the Asia-Pacific region in 2025, attributed to its massive population, expanding healthcare sector, and government-led immunization programs. Continuous investments in healthcare infrastructure and the modernization of hospitals and clinics are driving widespread adoption of safe medical devices. China’s strong domestic manufacturing capabilities also play a crucial role in improving the affordability and availability of auto disable syringes across urban and rural areas. Additionally, increasing awareness of injection safety, rising incidence of infectious diseases, and national initiatives focused on universal healthcare coverage continue to accelerate market growth in the country.
Auto Disable Syringes Market Share
The Auto Disable Syringes industry is primarily led by well-established companies, including:
• Terumo Corporation (Japan)
• Retractable Technologies, Inc. (U.S.)
• Numedico Technologies Pvt. Ltd. (India)
• Hindustan Syringes & Medical Devices Ltd. (India)
• Roma Medical Aids Pvt. Ltd. (India)
• Cardinal Health (U.S.)
• Vogt Medical Vertrieb GmbH (Germany)
• Nipro Corporation (Japan)
• Gerresheimer AG (Germany)
• Weigao Group (China)
• HLL Lifecare Limited (India)
• Smiths Medical (U.K.)
• Flexicare Medical Ltd. (U.K.)
• Baxter International Inc. (U.S.)
• Medtronic plc (Ireland)
• B. Braun Avitum (Germany)
• ICU Medical, Inc. (U.S.)
• Shandong Sinorgmed Co., Ltd. (China)
Latest Developments in Global Auto Disable Syringes Market
- In September 2023, Revital Healthcare EPZ Ltd. announced WHO pre-qualification / PQS status for its “early-activation” 0.5 ml auto-disable syringe, marking the first WHO-PQ acceptance for an African-made AD syringe and strengthening local supply capacity for immunization programs
- In February 2024, Gavi published a report highlighting that African-made auto-disable syringes (notably Revital’s product) were bolstering immunization capacity across the continent, underscoring the importance of regional manufacturing to close supply gaps
- In March 2024, Becton, Dickinson and Company (BD) publicly increased U.S. domestic syringe production (ramping Nebraska and Connecticut plants) in response to quality concerns over some imported syringes and to ensure continuity of supply for vaccination and clinical needs
- In March 2024, several Indian manufacturers (including Hindustan Syringes / HMD) announced launches and capacity expansions for safety/AD syringe lines — with public statements targeting production scales in the hundreds of millions per year to support both domestic immunization and export contracts
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.