South America IVD Regulatory Affairs Outsourcing Market to 2032
Overview
The South America IVD Regulatory Affairs Outsourcing Market is expected to reach a 0.10 USD Million by 2032 and is projected to grow at a CAGR of 17.66% from 2025 to 2032.
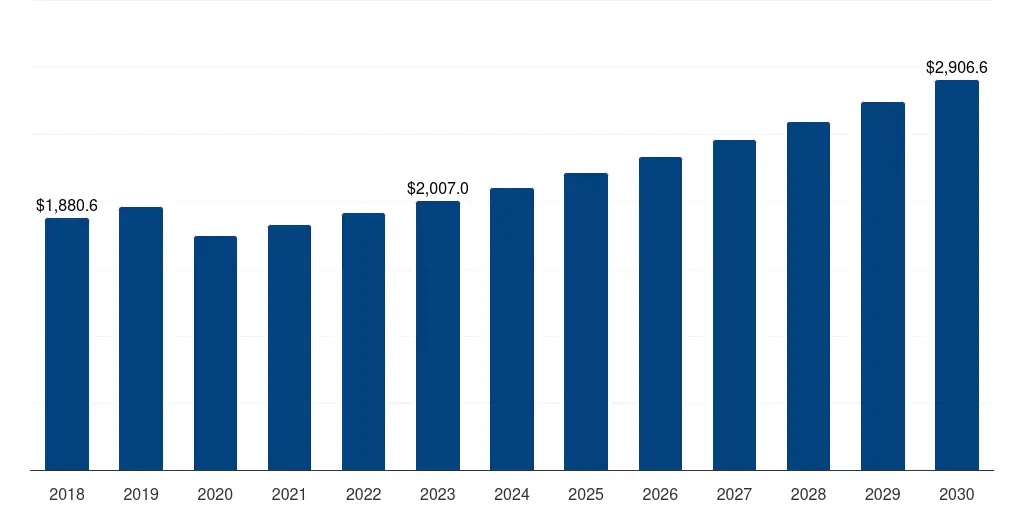
South America IVD Regulatory Affairs Outsourcing Market 2018-2032 USD Million
South America IVD Regulatory Affairs Outsourcing Market, Key Findings (2025-2032)
Market Growth and Projections:
- Market Size (2024): 0.04 USD Million
- Projected Market Size (2032): 0.10 USD Million
- CAGR (2025-2032): 17.66%
Key Findings of South America IVD Regulatory Affairs Outsourcing Market
- The South America IVD Regulatory Affairs Outsourcing Market was valued at 0.04 USD Million in 2024.
- The South America IVD Regulatory Affairs Outsourcing Market is likely to grow at a CAGR of 17.66% during the forecast period of 2024 to 2032.
- In 2024, the Largest segment Large Enterprises in Organization Size Segment accounted for the largest share of the market with a revenue of 0.02 USD Million
- The fastest growing segment Clinical Chemistry And Immunoassays in Indication Segment grew Fastest with a CAGR of 18.98% during the forecast period from 2024 to 2032.
South America IVD Regulatory Affairs Outsourcing Market Scope
- Others
- Genetic Testing
- HIV/AIDS
- Neurology
- Oncology
- Point Of Care
- Cardiology
- Blood Transfusion
- Diabetes
- Precision Medicine
- Drug Testing/Pharmacogenomics
- Haematology
- Infectious Diseases
- Clinical Chemistry And Immunoassays
- On-Premises
- Cloud
- Small & Medium Enterprises (SMEs)
- Large Enterprises
- PMA (Post Market Authorization)
- Preclinical
- Clinical
- Class II
- Class III
- Class I
- Others
- Biotechnology Companies
- Pharmaceutical Companies
- Medical Device Companies
- Others
- Data Management Services
- Chemistry Manufacturing And Controls (CMC) Services
- Regulatory Registration & Clinical Trial Applications
- Regulatory Consulting
- Legal Representation
- Regulatory Writing & Submissions
South America IVD Regulatory Affairs Outsourcing Market Data Coverage Insights
| Study Period | 2024-2032 |
| Base Year | 2021 |
| Unit | Revenue in USD Million |
| Market Value in 2024 | 0.04 USD Million |
| Market Value in 2032 | 0.10 USD Million |
| CAGR (2025-2032) | 17.66% |
| Historic Data | 2016-2023 |
| Market Segments Covered | Indication,Deployment Mode,Organization Size,Stage,Class,End User,Service |
Regional Insights:
-
Leading Market (2024-2032): South America, leading in terms of revenue 0.04 USD Million in 2024
- Key Country: Brazil, leading in terms of revenue with value of 0.02 USD Million in 2024.
Segments and Scope
-
South America IVD Regulatory Affairs Outsourcing Market to 2032, By Indication
- Clinical Chemistry And Immunoassays is the largest segment in South America IVD Regulatory Affairs Outsourcing Market to 2032 with a revenue of 0.01 USD Million in the year 2024.
- Clinical Chemistry And Immunoassays is the Fastest growing segment in South America IVD Regulatory Affairs Outsourcing Market to 2032 with a Growth rate of 18.98 % in forecast period 2025-2032.
-
South America IVD Regulatory Affairs Outsourcing Market to 2032, By Deployment Mode
- Cloud is the largest segment in South America IVD Regulatory Affairs Outsourcing Market to 2032 with a revenue of 0.02 USD Million in the year 2024.
- Cloud is the Fastest growing segment in South America IVD Regulatory Affairs Outsourcing Market to 2032 with a Growth rate of 18.51 % in forecast period 2025-2032.
-
South America IVD Regulatory Affairs Outsourcing Market to 2032, By Organization Size
- Large Enterprises is the largest segment in South America IVD Regulatory Affairs Outsourcing Market to 2032 with a revenue of 0.02 USD Million in the year 2024.
- Small & Medium Enterprises (SMEs) is the Fastest growing segment in South America IVD Regulatory Affairs Outsourcing Market to 2032 with a Growth rate of 16.96 % in forecast period 2025-2032.
-
South America IVD Regulatory Affairs Outsourcing Market to 2032, By Stage
- Clinical is the largest segment in South America IVD Regulatory Affairs Outsourcing Market to 2032 with a revenue of 0.02 USD Million in the year 2024.
- Clinical is the Fastest growing segment in South America IVD Regulatory Affairs Outsourcing Market to 2032 with a Growth rate of 18.64 % in forecast period 2025-2032.
-
South America IVD Regulatory Affairs Outsourcing Market to 2032, By Class
- Class I is the largest segment in South America IVD Regulatory Affairs Outsourcing Market to 2032 with a revenue of 0.02 USD Million in the year 2024.
- Class I is the Fastest growing segment in South America IVD Regulatory Affairs Outsourcing Market to 2032 with a Growth rate of 18.28 % in forecast period 2025-2032.
-
South America IVD Regulatory Affairs Outsourcing Market to 2032, By End User
- Medical Device Companies is the largest segment in South America IVD Regulatory Affairs Outsourcing Market to 2032 with a revenue of 0.02 USD Million in the year 2024.
- Medical Device Companies is the Fastest growing segment in South America IVD Regulatory Affairs Outsourcing Market to 2032 with a Growth rate of 18.36 % in forecast period 2025-2032.
-
South America IVD Regulatory Affairs Outsourcing Market to 2032, By Service
- Regulatory Writing & Submissions is the largest segment in South America IVD Regulatory Affairs Outsourcing Market to 2032 with a revenue of 0.02 USD Million in the year 2024.
- Legal Representation is the Fastest growing segment in South America IVD Regulatory Affairs Outsourcing Market to 2032 with a Growth rate of 18.15 % in forecast period 2025-2032.
South America IVD Regulatory Affairs Outsourcing Market Company Share Analysis
| Company Name |

|
||
| Medpace | |||
| Parexel International Corporation | |||
| ICON plc | |||
| LORENZ Life Sciences Group | |||
| MakroCare | |||

South America IVD Regulatory Affairs Outsourcing Market Geographical Sales Distribution, 2018-2032 USD Million
South America IVD Regulatory Affairs Outsourcing Market Company Profiling

Industry Related Reports
Frequently Asked Questions
South America IVD Regulatory Affairs Outsourcing Market Scope
- Others
- Genetic Testing
- HIV/AIDS
- Neurology
- Oncology
- Point Of Care
- Cardiology
- Blood Transfusion
- Diabetes
- Precision Medicine
- Drug Testing/Pharmacogenomics
- Haematology
- Infectious Diseases
- Clinical Chemistry And Immunoassays
- On-Premises
- Cloud
- Small & Medium Enterprises (SMEs)
- Large Enterprises
- PMA (Post Market Authorization)
- Preclinical
- Clinical
- Class II
- Class III
- Class I
- Others
- Biotechnology Companies
- Pharmaceutical Companies
- Medical Device Companies
- Others
- Data Management Services
- Chemistry Manufacturing And Controls (CMC) Services
- Regulatory Registration & Clinical Trial Applications
- Regulatory Consulting
- Legal Representation
- Regulatory Writing & Submissions
Frequently Asked Questions
South America IVD Regulatory Affairs Outsourcing Market Company Profiling

Frequently Asked Questions
HAVE A QUESTION?
Get expert advice on the right strategy for your business at no cost. We also offer customized subscription plans and affordable discounts for startups and universities.