U.S. Endotoxin And Pyrogen Testing Market to 2032
Overview
The U.S. Endotoxin And Pyrogen Testing Market is expected to reach a 1,543.33 USD Billion by 2032 and is projected to grow at a CAGR of 20.27% from 2025 to 2032.
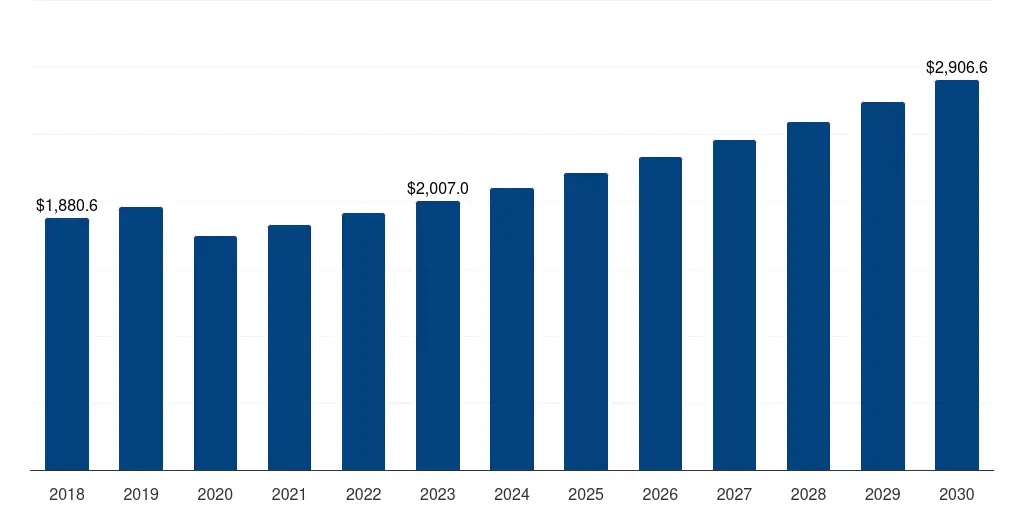
U.S. Endotoxin And Pyrogen Testing Market 2018-2032 USD Billion
U.S. Endotoxin And Pyrogen Testing Market, Key Findings (2025-2032)
Market Growth and Projections:
- Market Size (2024): 604.55 USD Billion
- Projected Market Size (2032): 1,543.33 USD Billion
- CAGR (2025-2032): 20.27%
Key Findings of U.S. Endotoxin And Pyrogen Testing Market
- The U.S. Endotoxin And Pyrogen Testing Market was valued at 604.55 USD Billion in 2024.
- The U.S. Endotoxin And Pyrogen Testing Market is likely to grow at a CAGR of 20.27% during the forecast period of 2024 to 2032.
- In 2024, the Largest segment Large Group in Mode of Purchase Segment accounted for the largest share of the market with a revenue of 371.06 USD Billion
- The fastest growing segment Pharmaceutical Companies in End User Segment grew Fastest with a CAGR of 22.37% during the forecast period from 2024 to 2032.
U.S. Endotoxin And Pyrogen Testing Market Scope
- Consumables & Accessories
- Endotoxin Testing Services
- Instruments, Systems and Softwares
- Detection Kits & Reagents
- Rabbit Pyrogen Test
- Recombinant C (RFC) Assay
- Monocyte Activation Test (MAT)
- TAL Tests
- Limulus Amoebocyte Lysate (LAL) Test
- Contract Manufacturing Organization (CMO)
- Contract Research Organization (CRO)
- Medical Device Companies
- Biomedical Devices
- Biotechnology Companies
- Pharmaceutical Companies
- Others
- Injectable
- Biologics
- Vaccines and/ Or CGT
- Individual
- Mid and Small Group
- Large Group
- Packaging Manufacture
- Raw Materials Production
- Medical Device Manufacturing
- Pharmaceutical Manufacturing
- Turbidimetric Endotoxin Test
- Chromogenic Endotoxin Test
- Gel Clot Endotoxin Test
U.S. Endotoxin And Pyrogen Testing Market Data Coverage Insights
| Study Period | 2024-2032 |
| Base Year | 2019 |
| Unit | Revenue in USD Billion |
| Market Value in 2024 | 604.55 USD Billion |
| Market Value in 2032 | 1,543.33 USD Billion |
| CAGR (2025-2032) | 20.27% |
| Historic Data | 2016-2023 |
| Market Segments Covered | Product Type,Test Type,End User,End Product,Mode of Purchase,Application,Method |
Regional Insights:
-
Leading Market (2024-2032): U.S., leading in terms of revenue 604.55 USD Billion in 2024
- Key Country: U.S., leading in terms of revenue with value of 604.55 USD Billion in 2024.
Segments and Scope
-
U.S. Endotoxin And Pyrogen Testing Market to 2032, By Product Type
- Detection Kits & Reagents is the largest segment in U.S. Endotoxin And Pyrogen Testing Market to 2032 with a revenue of 365.90 USD Billion in the year 2024.
- Detection Kits & Reagents is the Fastest growing segment in U.S. Endotoxin And Pyrogen Testing Market to 2032 with a Growth rate of 20.76 % in forecast period 2025-2032.
-
U.S. Endotoxin And Pyrogen Testing Market to 2032, By Test Type
- Limulus Amoebocyte Lysate (LAL) Test is the largest segment in U.S. Endotoxin And Pyrogen Testing Market to 2032 with a revenue of 348.01 USD Billion in the year 2024.
- Limulus Amoebocyte Lysate (LAL) Test is the Fastest growing segment in U.S. Endotoxin And Pyrogen Testing Market to 2032 with a Growth rate of 21.10 % in forecast period 2025-2032.
-
U.S. Endotoxin And Pyrogen Testing Market to 2032, By End User
- Pharmaceutical Companies is the largest segment in U.S. Endotoxin And Pyrogen Testing Market to 2032 with a revenue of 280.11 USD Billion in the year 2024.
- Pharmaceutical Companies is the Fastest growing segment in U.S. Endotoxin And Pyrogen Testing Market to 2032 with a Growth rate of 22.37 % in forecast period 2025-2032.
-
U.S. Endotoxin And Pyrogen Testing Market to 2032, By End Product
- Vaccines and/ Or CGT is the largest segment in U.S. Endotoxin And Pyrogen Testing Market to 2032 with a revenue of 367.69 USD Billion in the year 2024.
- Vaccines and/ Or CGT is the Fastest growing segment in U.S. Endotoxin And Pyrogen Testing Market to 2032 with a Growth rate of 21.46 % in forecast period 2025-2032.
-
U.S. Endotoxin And Pyrogen Testing Market to 2032, By Mode of Purchase
- Large Group is the largest segment in U.S. Endotoxin And Pyrogen Testing Market to 2032 with a revenue of 371.06 USD Billion in the year 2024.
- Large Group is the Fastest growing segment in U.S. Endotoxin And Pyrogen Testing Market to 2032 with a Growth rate of 20.83 % in forecast period 2025-2032.
-
U.S. Endotoxin And Pyrogen Testing Market to 2032, By Application
- Pharmaceutical Manufacturing is the largest segment in U.S. Endotoxin And Pyrogen Testing Market to 2032 with a revenue of 219.59 USD Billion in the year 2024.
- Pharmaceutical Manufacturing is the Fastest growing segment in U.S. Endotoxin And Pyrogen Testing Market to 2032 with a Growth rate of 21.39 % in forecast period 2025-2032.
-
U.S. Endotoxin And Pyrogen Testing Market to 2032, By Method
- Gel Clot Endotoxin Test is the largest segment in U.S. Endotoxin And Pyrogen Testing Market to 2032 with a revenue of 337.19 USD Billion in the year 2024.
- Chromogenic Endotoxin Test is the Fastest growing segment in U.S. Endotoxin And Pyrogen Testing Market to 2032 with a Growth rate of 19.92 % in forecast period 2025-2032.
U.S. Endotoxin And Pyrogen Testing Market Company Share Analysis
| Company Name |

|
||
| PALL CORPORATION | |||
| Lonza | |||
| THERMO FISHER SCIENTIFIC INC. | |||
| SGS Société Générale de Surveillance SA | |||
| CHARLES RIVER LABORATORIES | |||

U.S. Endotoxin And Pyrogen Testing Market Geographical Sales Distribution, 2018-2032 USD Billion
U.S. Endotoxin And Pyrogen Testing Market Company Profiling

Industry Related Reports
Frequently Asked Questions
U.S. Endotoxin And Pyrogen Testing Market Scope
- Consumables & Accessories
- Endotoxin Testing Services
- Instruments, Systems and Softwares
- Detection Kits & Reagents
- Rabbit Pyrogen Test
- Recombinant C (RFC) Assay
- Monocyte Activation Test (MAT)
- TAL Tests
- Limulus Amoebocyte Lysate (LAL) Test
- Contract Manufacturing Organization (CMO)
- Contract Research Organization (CRO)
- Medical Device Companies
- Biomedical Devices
- Biotechnology Companies
- Pharmaceutical Companies
- Others
- Injectable
- Biologics
- Vaccines and/ Or CGT
- Individual
- Mid and Small Group
- Large Group
- Packaging Manufacture
- Raw Materials Production
- Medical Device Manufacturing
- Pharmaceutical Manufacturing
- Turbidimetric Endotoxin Test
- Chromogenic Endotoxin Test
- Gel Clot Endotoxin Test
Frequently Asked Questions
U.S. Endotoxin And Pyrogen Testing Market Company Profiling

Frequently Asked Questions
HAVE A QUESTION?
Get expert advice on the right strategy for your business at no cost. We also offer customized subscription plans and affordable discounts for startups and universities.