During the forecast period, the lung cancer diagnostics market is expected to develop at a significant rate. The increase is mostly due to growing tobacco intake, particularly smoking. Tobacco use kills at least 6 million people per year, according to the World Health Organization (WHO). Because tobacco is the leading cause of lung cancer, the lung cancer diagnostics market is expected to rise. Other factors contributing to the rise in lung cancer patients include alcohol consumption, infection-carrying agents such as the human papillomavirus (HPV), pollution, and occupational carcinogens.
Access Full Report @ https://www.databridgemarketresearch.com/reports/global-non-small-cell-lung-cancer-diagnostics-market
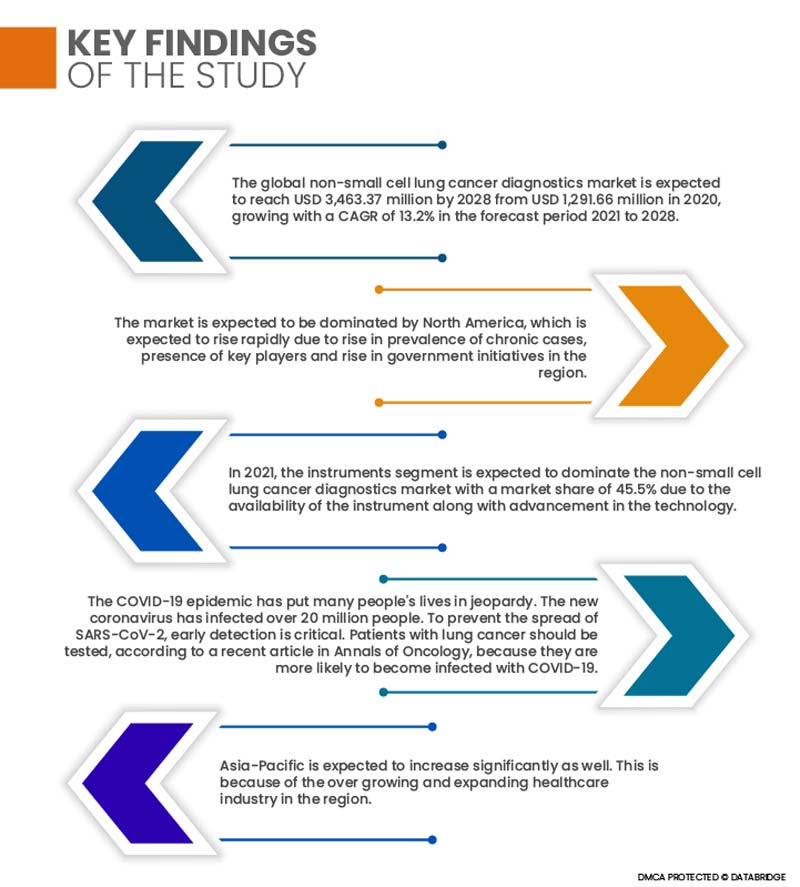
The global non-small cell lung cancer diagnostics market is expected to reach USD 3,463.37 million by 2028 from USD 1,291.66 million in 2020, growing with a CAGR of 13.2% in the forecast period 2021 to 2028. The demand for non-small cell lung cancer diagnostics is increasing, for which key players or companies are now more focused and are involved in the expansion, innovation, and publication of products in the non-small cell lung cancer diagnostics market. These decisions are ultimately enhancing the growth of the non-small cell lung cancer diagnostics market. Furthermore, rise in healthcare expenditure in developing countries and increase in adoption of new technology to diagnose non-small cell lung cancer will create huge opportunities for the global non-small cell lung cancer diagnostics market.
Technological advancements for the delivery of effective healthcare service will drive the market's growth rate
The improvements of the engineering applications to healthcare technologies also significantly impacted the country's economic growth. The emergent technologies, such as sensor technology, the internet of things (IoT), artificial intelligence (AI), big data and so on, have helped researchers and healthcare professionals design and develop efficient, innovative solutions in healthcare services to protect the workers from different hazards. This is driving the growth of the market. Increased expenditure by public and private players on infrastructure development will also promote the market growth rate. Continuous research and development pertaining to the deployment of advanced technologies will yet again widen the scope of growth.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2021 to 2028
|
|
Base Year
|
2020
|
|
Historic Years
|
2019 (Customizable to 2013 - 2018)
|
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Cancer Type (Lung Adenocarcinoma (LUAD), Lung Squamous Cell Carcinoma (LUSC), Large Cell Carcinoma And Others), Product (Reagents and Kits, Instruments, And Services And Softwares) Test(Imaging Test, Molecular Test, Biopsy, Sputum Cytology, Thoracentesis, Immunohistochemistry, And Others), End-User (Hospital, Clinical Laboratories, Academics And Others)
|
|
Countries Covered
|
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
|
|
Market Players Covered
|
Abbott Laboratories (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), Agilent Technologies, Inc. (U.S.), QIAGEN (Germany), Thermo Fisher Scientific Inc. (U.S.), Quest Diagnostics Incorporated (U.S.), NeoGenomics Laboratories, Inc. (U.S.), NanoString (U.S.), Janssen Pharmaceutical NV (U.S.), Inivata Ltd (U.K.), bioMérieux SA (France), Biotheranostics (U.S.), OncoCyte Corporation Inc. (U.S.), GENERAL ELECTRIC COMPANY (U.S.), Eckert & Ziegler (Germany), Dr. Lal PathLabs (India), RIVERAIN TECHNOLOGIES (U.S.), PlexBio Co., Ltd (Taiwan), Oncimmune (U.S.), Biodesix. (U.S.) among others.
|
|
Data Points Covered in the Report
|
In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The global non-small cell lung cancer diagnostics market is segmented into four notable segments based on the cancer type, products, test and end user.
- On the basis of cancer type, the global non small cell lung cancer diagnostics market is segmented into lung adenocarcinoma (LUAD), lung squamous cellcarcinoma (LUSC), large cell carcinoma and others. In 2021, the lung adenocarcinoma (LUAD) segment is expected to dominate the non-small cell lung cancer diagnostics market with a market share of 51.36% due to the high prevalence of Lung Adenocarcinoma among all types of non-small cell lung cancer.
Lung adenocarcinoma (LUAD) segment will dominate the cancer type segment of the non small cell lung cancer diagnostics market
The lung adenocarcinoma (LUAD) segment will emerge as the dominating segment under cancer type segment in 2021. This is because of the growing number of lung cancer cases all over the globe. Further, growth in the consumption rate of smoking products and other related tobacco products will further bolster the growth of this segment.
- On the basis of products, the global non-small cell lung cancer diagnostics market is segmented into reagents & kits, instruments and services & softwares. Reagents & kits are further segmented into lung cancer assay kit, sample preparation kit, mutation kit, solid tumor kit, extraction kit, and others. In 2021, the instruments segment is expected to dominate the non-small cell lung cancer diagnostics market with a market share of 45.5% due to the availability of the instrument along with advancement in the technology coupled with a rise in a number of launches of non-small cell lung cancer diagnostics instruments by the manufacturers.
- On the basis of test, the global non-small cell lung cancer diagnostics market is segmented imaging test, molecular test, biopsy, sputum cytology, thoracentesis, immunohistochemistry and others. In 2021, the molecular test segment is expected to dominate the non-small cell lung cancer diagnostics market with a market share of 51.46% due to the need for molecular tests to accurately and efficiently diagnose non-small cell lung cancer accurately and efficiently.
- On the basis of end user, the global non-small cell lung cancer diagnostics market is segmented into hospital, clinical laboratories, academics and institutes and research centers. In 2021, the clinical laboratories segment is expected to dominate the non-small cell lung cancer diagnostics market with a market share of 35.88% due to advancements in the technology used in the clinical laboratory to detect molecular abnormalities.
Clinical laboratories segment will dominate the end user segment of the non small cell lung cancer diagnostics market
The clinical laboratories will emerge as the dominating segment under distribution channel in 2021 with approximately 36% market share. This is because of the growing number of clinical laboratories in the market especially in the developing economies. Further, growth and expansion of the healthcare industry all around the globe will further bolster the growth of this segment.
Major Players
Data Bridge Market Research recognizes the following companies as the market players in non small cell lung cancer diagnostics market: Abbott Laboratories (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), Agilent Technologies, Inc. (U.S.), QIAGEN (Germany), Thermo Fisher Scientific Inc. (U.S.), Quest Diagnostics Incorporated (U.S.),), bioMérieux SA (France), Biotheranostics (U.S.), OncoCyte Corporation Inc. (U.S.), GENERAL ELECTRIC COMPANY (U.S.), Eckert & Ziegler (Germany), Dr. Lal PathLabs (India), Oncimmune (U.S.), Biodesix. (U.S.).
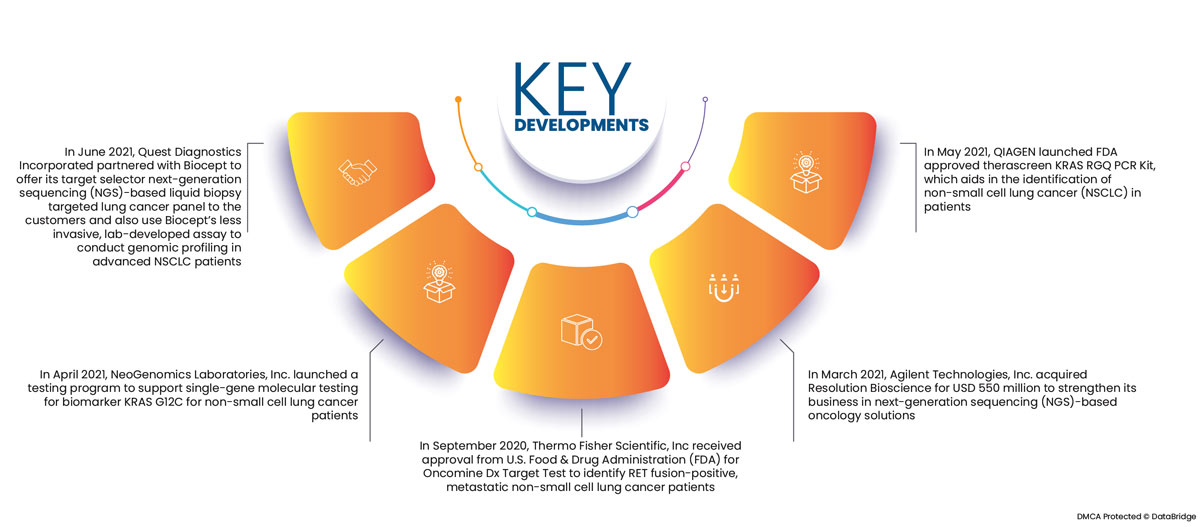
Market Development
- In June 2021, Quest Diagnostics Incorporated partnered with Biocept to offer its target selector next-generation sequencing (NGS)-based liquid biopsy targeted lung cancer panel to the customers and also use Biocept's less invasive, lab-developed assay to conduct genomic profiling in advanced NSCLC patients
- In April 2021, NeoGenomics Laboratories, Inc. launched a testing program to support single-gene molecular testing for biomarker KRAS G12C for non-small cell lung cancer patients
- In September 2020, Thermo Fisher Scientific, Inc received approval from U.S. Food & Drug Administration (FDA) for Oncomine Dx Target Test to identify RET fusion-positive, metastatic non-small cell lung cancer patients
- In March 2021, Agilent Technologies, Inc. acquired Resolution Bioscience for USD 550 million to strengthen its business in next-generation sequencing (NGS)-based oncology solutions
- In May 2021, QIAGEN launched FDA approved therascreen KRAS RGQ PCR Kit, which aids in the identification of non-small cell lung cancer (NSCLC) in patients
- In June 2020, F. Hoffmann-La Roche Ltd launched automated digitalpathology algorithm, uPath PD-L1 (SP263) image analysis to diagnose non-small cell lung cancer (NSCLC) and improve the speed and accuracy of diagnosis treatment
Regional Analysis
Geographically, the countries covered in the non small cell lung cancer diagnostics market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
As per Data Bridge Market Research analysis:
North America is the dominant region in non small cell lung cancer diagnostics market during the forecast period 2021 - 2028
The market is expected to be dominated by North America, which is expected to rise in prevalence of cancer cases, presence of key players, increase in healthcare expenditure, advancements in technology, and rise in government initiatives in the region. Due to a growth in the number of cancer patients and demand for new technologies, the United States was the largest market. In addition, the region's plasma fractionation market is fueled by the presence of major competitors.
Asia-Pacific is estimated to be the fastest-growing region in non small cell lung cancer diagnostics market the forecast period 2021 - 2028
Asia-Pacific is expected to increase significantly as well and will register the highest growth rate owing to an increase in the region's general population, a spike in facilities offering plasma fractionation services, and increased investments in the healthcare sector. Furthermore, as part of their economic growth aspirations, growing economies in APAC, such as India, are heavily investing in the infrastructure development.
COVID-19 Impact Analysis
The COVID-19 epidemic has put many people's lives in jeopardy. The new coronavirus has infected over 20 million people. To prevent the spread of SARS-CoV-2, early detection is critical. According to a recent article in Annals of Oncology, patients with lung cancer should be tested because they are more likely to become infected with COVID-19. As a result of this factor, the lung cancer diagnostics market will rise as more people test for early detection. Lung cancer testing businesses are also becoming involved with the COVID-19 testing process. Biodesix Inc., for example, has initiated tests for COVID-19 using the 'Droplet Digital PCR' test. Biodesix uses Droplet Digital to detect lung cancer. It is now being reviewed by the FDA.
For more detailed information about the non small cell lung cancer diagnostics on market report, click here – https://www.databridgemarketresearch.com/reports/global-non-small-cell-lung-cancer-diagnostics-market










