환각제 시장은 특히 정신 건강 및 치료 분야에서 관심과 활용이 다시금 높아지고 있습니다. 실로시빈과 MDMA와 같은 환각제는 우울증, 불안, 외상 후 스트레스 장애(PTSD), 중독 치료에 대한 잠재력을 연구하고 있습니다. 이 시장에서 가장 큰 비중을 차지하는 분야는 환각제 보조 치료(psychedelic-assisted therapy)로, 다양한 정신 건강 문제를 해결하기 위해 이러한 물질이 치료적 관행과 병행하여 사용됩니다. 이러한 전체론적 접근 방식은 정신 건강 관리에 혁신을 일으키고 전반적인 웰빙을 증진하는 데 유망한 것으로 보입니다.
https://www.databridgemarketresearch.com/reports/us-psychedelic-drugs-market 에서 전체 보고서를 확인하세요.
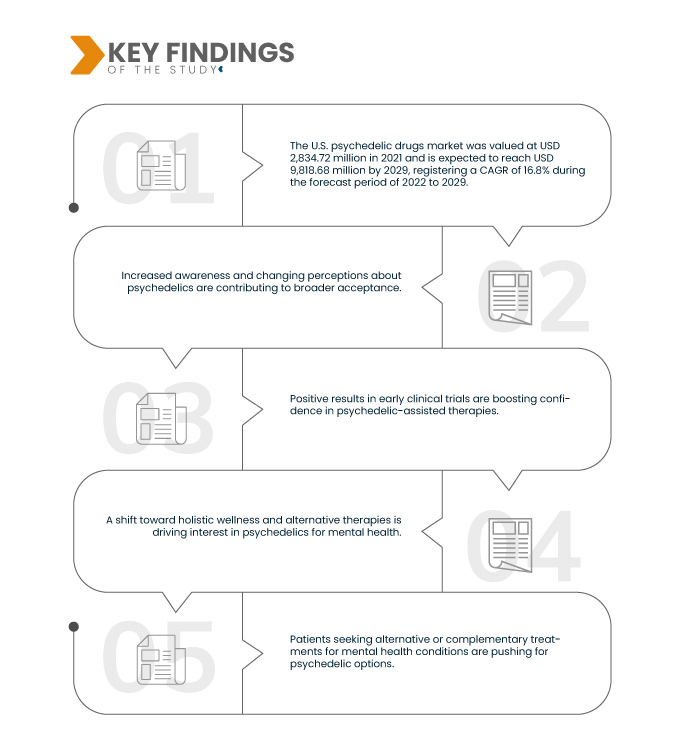
데이터 브리지 마켓 리서치(Data Bridge Market Research)에 따르면, 미국 환각제 시장은 2021년 28억 3,472만 달러 규모였으며, 2029년에는 98억 1,868만 달러에 도달하여 2022년부터 2029년까지 연평균 16.8%의 성장률을 기록할 것으로 예상됩니다. 우울증이나 외상 후 스트레스 장애(PTSD)와 같은 정신 건강 질환의 발병률이 증가함에 따라 혁신적인 치료법에 대한 수요가 더욱 커지고 있습니다. 기존 치료법은 한계가 있는 경우가 많아, 증가하는 정신 건강 위기를 해결하기 위해 환각제 보조 요법과 같은 새롭고 효과적인 치료법에 대한 수요가 증가하고 있습니다.
연구의 주요 결과
규제 변화로 시장 성장률이 높아질 것으로 예상
변화하는 규제는 환각제에 대한 인식과 연구 방식에 있어 주목할 만한 변화를 의미합니다. 이러한 변화 덕분에 연구자와 과학자들은 환각제를 포함한 더욱 광범위하고 엄격한 임상 시험을 수행할 수 있게 되었습니다. 규제 완화는 정신 건강 장애 치료에 있어 이러한 물질의 치료적 잠재력을 탐구할 수 있는 기회를 제공했습니다. 이러한 규제 변화는 혁신적인 해결책의 필요성을 인정하고, 통제되고 안전한 체계 내에서 환각제의 유망한 효능을 밝혀내기 위한 과학적 탐구를 장려합니다.
보고서 범위 및 시장 세분화
보고서 메트릭
|
세부
|
예측 기간
|
2022년부터 2029년까지
|
기준 연도
|
2021
|
역사적인 해
|
2020 (2014-2019년으로 맞춤 설정 가능)
|
양적 단위
|
매출(백만 달러), 볼륨(단위), 가격(달러)
|
다루는 세그먼트
|
출처(합성, 천연), 유형(감마하이드록시뷰티르산, 케타민, 실로시빈, 기타), 약물(감마하이드록시뷰티르산, 케타민, 실로시빈, 기타), 적용 분야(발작성 졸음증, 치료 저항성 우울증, 주요 우울 장애, 아편 중독, 외상 후 스트레스 장애 , 기타), 투여 경로(경구, 흡입, 주사), 최종 사용자(병원, 전문 클리닉, 재택 치료, 기타), 유통 채널(병원 약국, 소매 약국, 온라인 약국)
|
포함 국가
|
북미에서는 미국, 캐나다, 멕시코가 있습니다.
|
시장 참여자 포함
|
Avadel(아일랜드), Celon Pharma SA(폴란드), Johnson & Johnson Private Limited(미국), Hikma Pharmaceuticals PLC(영국), Amneal Pharmaceuticals LLC.(미국), NeuroRx, Inc.(미국), Jazz Pharmaceuticals, Inc.(아일랜드), COMPASS(영국), Develco Pharma Schweiz AG(스위스), Douglas Pharmaceuticals Limited(뉴질랜드)
|
보고서에서 다루는 데이터 포인트
|
Data Bridge Market Research에서 큐레이팅한 시장 보고서에는 시장 가치, 성장률, 세분화, 지리적 적용 범위, 주요 기업 등 시장 시나리오에 대한 통찰력 외에도 심층적인 전문가 분석, 환자 역학, 파이프라인 분석, 가격 분석, 규제 프레임워크가 포함됩니다.
|
세그먼트 분석:
미국의 환각제 시장은 출처, 유형, 약물, 용도, 투여 경로, 최종 사용자 및 유통 채널을 기준으로 세분화됩니다.
- 미국의 환각제 시장은 출처를 기준으로 합성 환각제와 천연 환각제로 구분됩니다.
- 미국의 환각제 시장은 유형을 기준으로 공감제, 해리제 및 기타로 구분됩니다.
- 미국의 환각제 시장은 약물을 기준으로 감마-하이드록시뷰티르산, 케타민, 실로시빈 등으로 구분됩니다.
- 미국의 환각제 시장은 응용 분야를 기준으로 기면증, 치료 저항성 우울증, 주요 우울 장애, 아편 중독, 외상 후 스트레스 장애 등으로 구분됩니다.
- 투여 경로를 기준으로 미국의 환각제 시장은 경구용, 흡입용, 주사용으로 구분됩니다.
- 미국의 환각제 시장은 최종 사용자를 기준으로 병원, 전문 클리닉, 재택 치료 및 기타로 구분됩니다.
- 유통 채널을 기준으로 볼 때, 미국의 환각제 시장은 병원 약국, 소매 약국, 온라인 약국으로 구분됩니다.
주요 플레이어
Data Bridge Market Research에서는 미국 환각제 시장의 주요 기업으로 Avadel(아일랜드), Celon Pharma SA(폴란드), Johnson & Johnson Private Limited(미국), Hikma Pharmaceuticals PLC(영국), Amneal Pharmaceuticals LLC.(미국), NeuroRx, Inc.(미국), Jazz Pharmaceuticals, Inc.(아일랜드), COMPASS(영국)를 꼽았습니다.
시장 개발
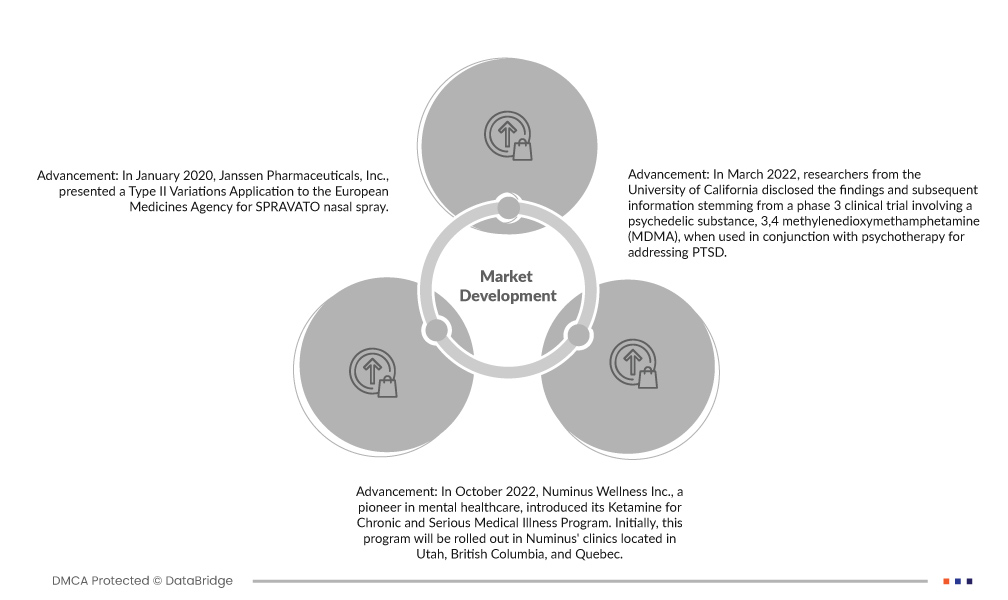
- 2020년 1월, 얀센 파마슈티컬스(Janssen Pharmaceuticals, Inc.)는 유럽 의약품청(EMA)에 SPRAVATO 비강 스프레이에 대한 제2종 변경 허가 신청서를 제출했습니다. 이 신청서는 더 광범위한 환자들의 우울증 증상 관리를 위한 단기 급성 치료제로서 비강 스프레이의 활용을 확대하기 위한 것입니다. 이러한 전략적 조치는 제품의 시장 도달 범위를 확대하고 회사의 매출을 증대시키는 것을 목표로 합니다.
- 2022년 10월, 정신 건강 분야의 선구자인 Numinus Wellness Inc.는 만성 및 중증 질환 환자를 위한 케타민 프로그램을 출시했습니다. 이 프로그램은 우선 유타주, 브리티시컬럼비아주, 퀘벡주에 위치한 Numinus 클리닉에서 시행될 예정입니다. Numinus는 향후 몇 달 안에 더 많은 클리닉으로 프로그램을 점진적으로 확대할 계획이며, 이는 만성 및 중증 질환 환자를 위한 혁신적이고 근거 기반의 환각 보조 치료법을 발전시키겠다는 Numinus의 의지를 보여줍니다.
- 2022년 3월, 캘리포니아 대학교 연구진은 외상 후 스트레스 장애(PTSD) 치료를 위해 심리 치료와 병행하여 환각제인 3,4-메틸렌디옥시메탐페타민(MDMA)을 사용한 3상 임상 시험 결과와 그 결과를 공개했습니다. 초기 데이터는 약물 또는 알코올 사용 장애 병력이 있는 환자를 포함하여 까다로운 환자군에서도 MDMA 치료의 효능을 보여주었습니다. 이는 이러한 복합적 접근법이 외상 후 스트레스 장애(PTSD) 치료에 유망한 잠재력을 가지고 있음을 시사합니다.
미국 환각제 시장 보고서 에 대한 자세한 내용은 여기를 클릭하세요 - https://www.databridgemarketresearch.com/reports/us-psychedelic-drugs-market