The importance of secondary hyperoxaluria drugs lies in their potential to prevent kidney damage and mitigate the progression of chronic kidney disease. It targets the underlying causes or mechanisms of oxalate overproduction; these drugs offer a proactive approach to preserving renal function. Preventing or slowing down kidney damage enhances the quality of life for individuals with secondary hyperoxaluria and reduces the risk of more severe complications associated with advanced kidney disease.
Access Full Report @ https://www.databridgemarketresearch.com/reports/us-secondary-hyperoxaluria-drug-market
Data Bridge Market Research analyses a growth rate in the U.S. Secondary Hyperoxaluria Drug Market in the forecast period 2022-2029. The heightened awareness of secondary hyperoxaluria in the U.S. serves as a key driver for the drug market. As understanding of the condition increases, more cases are expected to be diagnosed, expanding the patient base in need of treatment and thereby driving demand for secondary hyperoxaluria drugs.
Key Findings of the Study
Increasing patient advocacy groups is expected to drive the market's growth rate
Patient advocacy groups focusing on secondary hyperoxaluria play a pivotal role in driving growth in the U.S. drug market for this condition. These organizations contribute significantly to increased awareness among both patients and healthcare professionals, fostering early diagnosis and treatment. It actively engages in advocacy efforts; these groups also attract research funding, accelerating the development of innovative therapies. Furthermore, their involvement in policy initiatives can influence regulatory frameworks, potentially expediting the approval process for new drugs.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2022 to 2029
|
|
Base Year
|
2021
|
|
Historic Years
|
2020 (Customizable to 2014-2019)
|
|
Segments Covered
|
Type (Reloxaliase, Thiazide Diuretics and Supplements), Drug Type (Prescription and Over the Counter), Population Type (Children and Adults), End-User (Hospitals, Homecare, Specialty Clinics, Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, Retail Pharmacy)
|
|
Market Players Covered
|
Nestlé (Switzerland), GSK plc (U.K.), Bayer AG (Germany), Pharmavite (U.S.), Mission Pharmacal Company (U.S.), Oystershell (Belgium), Allena Pharmaceuticals (U.S.), OxThera (Sweden), Renew Life (U.S.), Synlogic (U.K.), Amway Corp (U.S.), Infinitus (China), Nature’s Bounty (Bohemia), Now Foods (Canada), Solgar Inc. (U.S.)
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The U.S. secondary hyperoxaluria drug market is segmented on the basis of type, drug type, population type, distribution channel, and end-user.
- On the basis of type, the U.S. secondary hyperoxaluria drug market is segmented into reloxaliase, thiazide diuretics, and supplements
- On the basis of drug type, the U.S. secondary hyperoxaluria drug market is segmented into prescription and over the counter
- On the basis of population type, the U.S. secondary hyperoxaluria drug market is segmented into children and adults
- On the basis of distribution channel, the U.S. secondary hyperoxaluria drug market is segmented into hospitals, homecare, specialty clinics, and others
- On the basis of end-user, the U.S. secondary hyperoxaluria drug market is segmented into hospital pharmacy, online pharmacy, and retail pharmacy
Major Players
Data Bridge Market Research recognizes the following companies as the major U.S. Secondary hyperoxaluria drug market players in U.S. secondary hyperoxaluria drug market is Nestlé (Switzerland), GSK plc (U.K.), Bayer AG (Germany), Pharmavite (U.S.), Mission Pharmacal Company (U.S.), Oystershell (Belgium), Allena Pharmaceuticals (U.S.), OxThera (Sweden)
Market Developments
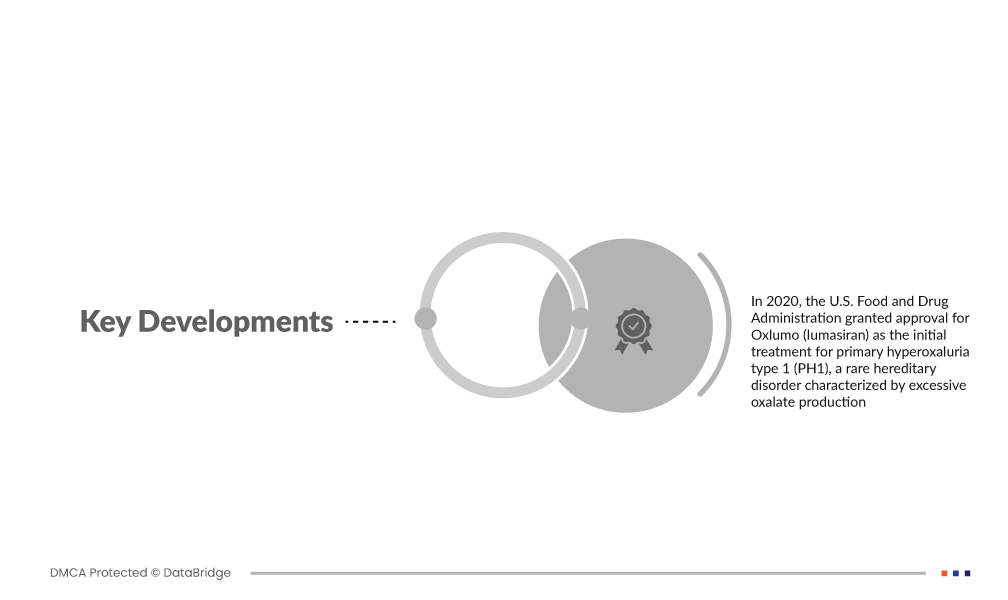
- In 2020, the U.S. Food and Drug Administration granted approval for Oxlumo (lumasiran) as the initial treatment for primary hyperoxaluria type 1 (PH1), a rare hereditary disorder characterized by excessive oxalate production. This milestone approval resulted from collaborative efforts involving specialists, researchers, and patient communities, particularly led by the Oxalosis & Hyperoxaluria Foundation and the Kidney Health Initiative. The green light for Oxlumo represents a significant advancement in addressing the genetic root of PH1, offering a targeted therapeutic option and highlighting the impact of patient advocacy and collaborative research in rare diseases. This approval signals a transformative shift in the management of PH1, showcasing the potential of precision medicine to address the underlying causes of rare genetic disorders
For more detailed information about the U.S. secondary hyperoxaluria drug market report, click here – https://www.databridgemarketresearch.com/reports/us-secondary-hyperoxaluria-drug-market