Europe Diagnostic Tests Market
Market Size in USD Million
CAGR :
% 
 USD
160,573.05 Million
USD
324,678.67 Million
2022
2030
USD
160,573.05 Million
USD
324,678.67 Million
2022
2030
| 2023 –2030 | |
| USD 160,573.05 Million | |
| USD 324,678.67 Million | |
|
|
|
|
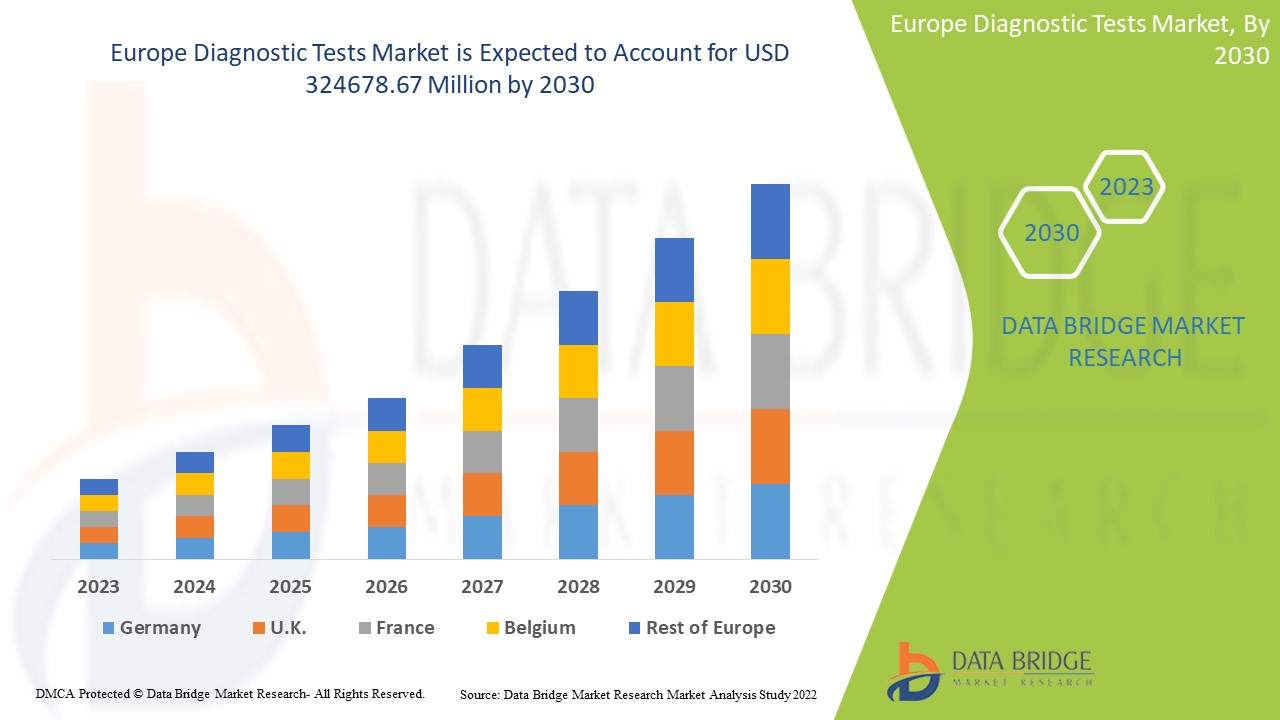
Europe Diagnostic Tests Market Analysis and Size
According to the World Health Organization (WHO), chronic diseases will account for approximately 41 million deaths per year by April 2021, accounting for 71% of all fatalities. As a result, diagnostic tests have proven to be beneficial in the management of chronic illness conditions, as well as disease prevention, detection, and diagnosis. By identifying individual risk factors and early warning indicators, clinical diagnostics enable early prevention and intervention. As a result, as the prevalence of chronic illnesses rises, the overall market is expected to grow.
Data Bridge Market Research analyses that the diagnostic tests market, which was USD 160,573.05 million in 2022, is expected to reach USD 324678.67 million by 2030, at a CAGR of 9.2% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Europe Diagnostic Tests Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Type (Glucose Test, Infectious Diseases Test, Cytology Test, CBC Test, Blood Culture Test, Syphilis Test, Urea Test, C-Reactive Protein Test, Antigen Test, HBA1C Test, Pregnancy Test, Lipid Profile Test, Electrolytes Test, Liver Function Test, Stool Helicobacter Pylori Test, Calcium Test, Crossmatch Test, Thyroid Function Test, Stool Microscopy Test, Urine Microscopy Test, Unit Packed RBCS Test, ESR Test and Others Test), Solutions (Services and Products), Technology (Immunoassay-Based, PCR-Based, Next Gene Sequencing, Spectroscopy-Based, Chromatography-Based, Microfluidics, Substrate Technology and Others), Mode of Testing (Prescription-Based Testing and OTC Testing), Approach (Molecular Diagnostic Instrument, In-Vitro Diagnostic Instrument and Point Of Care Testing Instrument), Sample Type (Urine, Saliva, Blood, Hair, Sweat and Others), Application (Cardiology, Oncology, Neurology, Orthopedics, Gastroenterology, Gynecology, Odontology and Others), Testing Type (Biochemistry, Haematology, Microbiology, Histopathology and Others), Age (Pediatric, Adult and Geriatric), End User (Hospitals, Diagnostic Center, Research Labs and Institutes, Research Institute, Homecare, Blood Banks, Specialty Clinics, Ambulatory Surgical Centers and Others), Distribution Channel (Direct Tenders, Retail Sales and Online Sales) |
|
Countries Covered |
Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe |
|
Market Players Covered |
F-Hoffman La-Roche Ltd. (Switzerland), ABBOTT (U.S.), Danaher (U.S.), B.D. (U.S.), Thermo Fisher Scientific Inc. (U.S.), ACON Laboratories Inc. (U.S.), Hemosure, Inc. (U.S.), MicroGen Diagnostics (U.S.), QIAGEN (Germany), Grifols, S.A (Spain), BODITECH MED INC. (South Korea), Chembio Diagnostic Systems, Inc. (U.S.), Nanoentek (South Korea), DiaSorin S.p.A. (Italy), Bio-Rad Laboratories, Inc. (U.S.), BIOMEDOMICS INC (U.S.), EKF Diagnostics Holdings plc (U.K.), Siemens Healthcare GmbH (Germany), PerkinElmer Inc. (U.S.), BIOMÉRIEUX (France), ARKRAY USA, Inc. (U.S.), Biohit Oyj (Finland), Germaine Laboratories, Inc. (U.S.), Quidel Corporation (U.S.), Illumina, Inc. (U.S.), Lamdagen Corporation (U.S.), LifeSign LLC. (U.S.), Medixbiochemica (Finland), Nova Biomedical (U.S.), Ortho Clinical Diagnostics (U.S.), Sannuo Biosensing Co., Ltd. (U.S.), STRECK (U.S.), Sysmex Corporation (Japan), among others |
|
Market Opportunities |
|
Market Definition
Diagnostic tests are medical procedures used to aid in the detection or diagnosis of disease. These tests play an important role in disease control, surveillance, and prevention. These tests aid in the enhancement of patient care, consumer safety, and healthcare spending. A diagnostic test is one that is used to figure out what is causing a condition. A diagnostic test performed as part of a medical examination may be used to identify a disease or to determine the cause of symptoms. When used for other purposes, a diagnostic test can be used to identify specific strengths and weaknesses.
Europe Diagnostic Tests Market Dynamics
Drivers
- Growing incidences of infectious and chronic diseases
Infectious diseases and chronic conditions are putting a strain on the global population. Infectious diseases like diphtheria, ebola, flu, hepatitis, HIV/AIDS, HPV, tuberculosis, and others are caused by microorganisms, and sudden outbreaks like dengue, Zika virus, COVID-19, and Swine flu drive international demand for clinical diagnostic tests. Furthermore, chronic diseases such as cancer, diabetes, cardiovascular disease, obesity, and others are driving up demand for clinical diagnostics.
- Rising clinical diagnostic tests
The most accurate techniques for identifying and describing microorganisms are clinical diagnostic tests. A practical test needs to be quick, precise, and able to identify the infection's severity. The time it takes to locate the proper antibiotic is decreased through faster, more accurate testing that identifies the strain of the organism and its medication susceptibility. According to a World Health Organization (WHO) report, tuberculosis (TB) was one of the top ten causes of death. This will probably have a big impact on market expansion. The rising prevalence of cancer cases in living standards can be largely attributed to the accessibility and availability of better diagnostic techniques. Because clinical cancer diagnostics equipment is widely used in these procedures, demand for diagnostic tests rises during the forecast period.
Opportunities
- Growing demand for advanced diagnostic solutions
Clinical diagnostics are used to manage patients' health. It allows for the earlier detection of diseases and aids in the progression of illnesses. Furthermore, it may aid infected individuals in avoiding long-term consequences, raising public awareness of the significance of clinical diagnosis. Increased healthcare spending and health awareness encourage market growth. As people grow more concerned about the possibility of disease transmission from infected patients, the demand for clinical diagnostic kits is anticipated to increase during the forecast period. Diagnostic testing can be carried out using services as well as equipment and supplies. This feature will almost certainly improve patient comfort, which will benefit the market.
Restraints/Challenges
- Affordability for diagnostics tests
Pricing pressures from reimbursement cuts and budget constraints, along with stringent regulatory policies, are expected to hamper market growth. Furthermore, the diagnostic tests market is expected to be challenged by a lack of alignment with definitive central lab methods and insufficient adoption of POC devices in professional settings during the forecast period of 2023-2030.
This diagnostic tests market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the diagnostic tests market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on the Diagnostic Tests Market
COVID-19 has negatively impacted the diagnostic test market because lab testing has increased, causing demand to rise even faster to keep up with suspected COVID-19 cases. According to the Atlantic Monthly Group, the number of COVID-19 tests worldwide increased dramatically from 760,441 in September 2020 to 964,792 in October 2020. As a result, the rising number of tests due to increased patient numbers and government funding is expected to drive demand for COVID-19 test kits and fuel the overall market's exponential growth.
Recent Developments
- In 2020, Siemens Healthcare GmbH officially announced the release of the RAPIDPoint 500e Blood Gas Analyzer, which broadened the company's product portfolio and is also used in COVID-19 efforts. This helps to increase revenue from the line.
- In 2020, Siemens Healthcare GmbH and the Marienhaus Hospital Group have announced a ten-year collaboration. This collaboration will benefit the company in diagnostics in the long run. This agreement will also help the company's financial situation.
Europe Diagnostic Tests Market Scope
The diagnostic tests market is segmented on the basis of type, solution, technology, mode of testing, approach, sample type, application, testing type, age, end user and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Glucose Test
- Infectious Diseases Test
- Cytology Test
- CBC Test
- Blood Culture Test
- Syphilis Test
- Urea Test
- C-Reactive Protein Test
- Antigen Test
- HBA1C Test
- Pregnancy Test
- Lipid Profile Test
- Electrolytes Test
- Liver Function Test
- Stool Helicobacter Pylori Test
- Calcium Test, Crossmatch Test
- Thyroid Function Test
- Stool Microscopy Test
- Urine Microscopy Test
- Unit Packed Rbcs Test
- ESR Test and Others Test
Solutions
- Services
- Products
Technology
- Immunoassay-Based
- PCR-Based
- Next Gene Sequencing
- Spectroscopy-Based
- Chromatography-Based
- Microfluidics
- Substrate Technology
- Others
Mode of Testing
- Prescription-Based Testing
- OTC Testing
Approach
- Molecular Diagnostic Instrument
- In-Vitro Diagnostic Instrument
- Point of Care Testing Instrument
Sample Type
- Urine
- Saliva
- Blood
- Hair
- Sweat
- Others
Application
- Cardiology
- Oncology
- Neurology
- Orthopedics
- Gastroenterology
- Gynecology
- Odontology
- Others
Testing Type
- Biochemistry
- Hematology
- Microbiology
- Histopathology
- Others
Age
- Pediatric
- Adult
- Geriatric
End User
- Hospitals
- Diagnostic Center
- Research Labs and Institutes
- Research Institute
- Homecare
- Blood Banks
- Specialty Clinics
- Ambulatory Surgical Centers
- Others
Distribution Channel
- Direct Tenders
- Retail Sales
- Online Sales
Europe Diagnostic Tests Market Regional Analysis/Insights
The diagnostic tests market is analyzed and market size insights and trends are provided by country, type, solution, technology, mode of testing, approach, sample type, application, testing type, age, end user and distribution channel as referenced above.
The countries covered in the diagnostic tests market report are Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe.
Germany is dominating the Europe diagnostic tests market to government strategic initiatives such as acquisition and focused segment product launches, which are assisting them to expand their reach and also assisting them to expand and enhance the company's product portfolio, which will ultimately lead to more revenue generation in the country.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Diagnostic Tests Market Share Analysis
The diagnostic tests market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to diagnostic tests market.
Some of the major players operating in the diagnostic tests market are:
- F-Hoffman La-Roche Ltd. (Switzerland)
- ABBOTT (U.S.)
- Danaher (U.S.)
- B.D. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- ACON Laboratories Inc. (U.S.)
- Hemosure, Inc. (U.S.)
- MicroGen Diagnostics (U.S.)
- QIAGEN (Germany)
- Grifols, S.A (Spain)
- BODITECH MED INC. (South Korea)
- Chembio Diagnostic Systems, Inc. (U.S.)
- Nanoentek (South Korea)
- DiaSorin S.p.A. (Italy)
- Bio-Rad Laboratories, Inc. (U.S.)
- BIOMEDOMICS INC (U.S.)
- EKF Diagnostics Holdings plc (U.K.)
- Siemens Healthcare GmbH (Germany)
- PerkinElmer Inc. (U.S.)
- BIOMÉRIEUX (France)
- ARKRAY USA, Inc. (U.S.)
- Biohit Oyj (Finland)
- Germaine Laboratories, Inc. (U.S.)
- Quidel Corporation (U.S.)
- Illumina, Inc. (U.S.)
- Lamdagen Corporation (U.S.)
- LifeSign LLC. (U.S.)
- Medixbiochemica (Finland)
- Nova Biomedical (U.S.)
- Ortho Clinical Diagnostics (U.S.)
- Sannuo Biosensing Co., Ltd. (U.S.)
- STRECK (U.S.)
- Sysmex Corporation (Japan)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE DIAGNOSTICS TESTS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE EUROPE DIAGNOSTICS TESTS MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES VOLUME DATA
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 EUROPE DIAGNOSTICS TESTS MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER'S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNOLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 FUTURE OUTLOOK
11 REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.2.1 CLASS I
11.2.2 CLASS II
11.2.3 CLASS III
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
12 REIMBURSEMENT FRAMEWORK
13 OPPUTUNITY MAP ANALYSIS
14 INSTALLED BASE DATA
15 VALUE CHAIN ANALYSIS
16 HEALTHCARE ECONOMY
16.1 HEALTHCARE EXPENDITURE
16.2 CAPITAL EXPENDITURE
16.3 CAPEX TRENDS
16.4 CAPEX ALLOCATION
16.5 FUNDING SOURCES
16.6 INDUSTRY BENCHMARKS
16.7 GDP RATION IN OVERALL GDP
16.8 HEALTHCARE SYSTEM STRUCTURE
16.9 GOVERNMENT POLICIES
16.1 ECONOMIC DEVELOPMENT
17 EUROPE DIAGNOSTICS TESTS MARKET, BY TYPE
17.1 OVERVIEW
17.2 ROUTINE TESTS
17.2.1 GLUCOSE TEST
17.2.2 INFECTIOUS DISEASES TEST
17.2.3 CBC TEST
17.2.4 BLOOD CULTURE TEST
17.2.5 SYPHILIS TEST
17.2.6 UREA TEST
17.2.7 C-REACTIVE PROTEIN TEST
17.2.8 ANTIGEN TEST
17.2.9 HBA1C TEST
17.2.10 PREGNANCY TEST
17.2.11 LIPID PROFILE TEST
17.2.12 ELECTROLYTES TEST
17.2.13 LIVER FUNCTION TEST
17.2.14 STOOL HELICOBACTER PYLORI TEST
17.2.15 CALCIUM TEST
17.2.16 CROSSMATCH TEST
17.2.17 THYROID FUNCTION TEST
17.2.18 STOOL MICROSCOPY TEST
17.2.19 URINE MICROSCOPY TEST
17.2.20 UNIT PACKED RBCS TEST
17.2.21 ESR TEST
17.2.22 OTHERS
17.3 SPECIALIZED TESTS
17.3.1 MOLECULAR DIAGNOSTIC TESTS
17.3.2 IMMUNOLOGICAL TESTS
17.3.3 GENETIC TESTS
17.3.4 TOXICOLOGY TESTS
17.3.5 OTHERS
18 EUROPE DIAGNOSTICS TESTS MARKET, BY COMPONENT
18.1 OVERVIEW
18.2 PRODUCTS
18.2.1 INSTRUMENTS
18.2.1.1. BY TYPE
18.2.1.1.1. IN-VITRO DIAGNOSTIC INSTRUMENT
18.2.1.1.1.1 IMMUNOASSAY INSTRUMENT
18.2.1.1.1.1.1. BY TYPE
A. ELISA READERS
B. CLIA ANALYZERS
C. OTHERS
18.2.1.1.1.1.2. BY MODE
A. AUTOMATED
B. SEMI-AUTOMATED
C. MANUAL
18.2.1.1.1.1.3. BY MODALITY
A. BENCHTOP
B. FLOOR-STANDING
C. MODULAR
18.2.1.1.1.2 CLINICAL CHEMISTRY INSTRUMENT
18.2.1.1.1.2.1. BY TYPE
A. SPECTROPHOTOMETERS
B. ELECTROLYTE ANALYZERS
C. BLOOD GAS ANALYZERS
D. COAGULATION ANALYZERS
E. MICROPLATE READERS
18.2.1.1.1.2.2. BY MODE
A. AUTOMATED
B. SEMI-AUTOMATED
C. MANUAL
18.2.1.1.1.2.3. BY MODALITY
A. BENCHTOP
B. FLOOR-STANDING
C. MODULAR
18.2.1.1.1.3 HEMATOLOGY INSTRUMENT
18.2.1.1.1.3.1. BY MODE
A. AUTOMATED
B. SEMI-AUTOMATED
C. MANUAL
18.2.1.1.1.3.2. BY MODALITY
A. BENCHTOP
B. FLOOR-STANDING
C. MODULAR
18.2.1.1.1.4 MOLECULAR DIAGNOSTIC INSTRUMENT
18.2.1.1.1.4.1. BY MODE
A. PCR INSTRUMENTS
B. NGS INSTRUMENTS
C. MICROARRAY INSTRUMENTS
D. ISOTHERMAL AMPLIFICATION INSTRUMENTS
E. NUCLEIC ACID EXTRACTION INSTRUMENTS
F. HYBRIDIZATION INSTRUMENTS
18.2.1.1.1.4.2. BY MODE
A. AUTOMATED
B. SEMI-AUTOMATED
C. MANUAL
18.2.1.1.1.4.3. BY MODALITY
A. BENCHTOP
B. FLOOR-STANDING
C. MODULAR
18.2.1.1.1.5 OTHERS
18.2.1.1.2. POINT OF CARE TESTING INSTRUMENT
18.2.1.1.2.1 BLOOD GLUCOSE MONITORING DEVICES
18.2.1.1.2.2 RAPID DIAGNOSTIC TEST DEVICES
18.2.1.1.2.3 CARDIAC MARKER DEVICES
18.2.1.1.2.4 INFECTIOUS DISEASE TESTING DEVICES
18.2.1.1.2.5 URINE ANALYSIS DEVICES
18.2.1.2. OTHER
18.2.2 KITS AND REAGENTS
18.2.2.1. PREGNANCY TESTING KITS
18.2.2.1.1. STRIPS
18.2.2.1.2. CASETTES
18.2.2.1.3. MIDSTREAM
18.2.2.1.4. DIP CARD
18.2.2.1.5. OTHERS
18.2.2.2. URINALYSIS TESTING KITS
18.2.2.2.1. CASSETTES TESTS
18.2.2.2.2. STRIP TESTS
18.2.2.3. GLUCOSE MONITORING KITS
18.2.2.3.1. STRIPS
18.2.2.3.2. CARTRIDGES
18.2.2.4. CARDIAC ASSAYS
18.2.2.4.1. TROPONIN
18.2.2.4.2. BNP
18.2.2.4.3. MYOGLOBIN
18.2.2.4.4. OTHERS
18.2.2.5. INFECTIOUS DISEASE TESTING KITS
18.2.2.5.1. HEPATITIS
18.2.2.5.2. INFLUENZA
18.2.2.5.3. HIV/AIDS
18.2.2.5.4. TUBERCULOSIS
18.2.2.5.5. HOSPITAL ACQUIRED INFECTIONS
18.2.2.5.6. COVID-19
18.2.2.5.7. OTHERS
18.2.2.6. DRUG TEST KITS
18.2.2.6.1. MARIJUANA
18.2.2.6.2. AMPHETAMINES
18.2.2.6.3. BARBITUATES
18.2.2.6.4. ANTI DEPRESSANTS
18.2.2.6.5. PCP
18.2.2.6.6. METHADONE
18.2.2.6.7. OTHERS
18.2.2.7. OTHERS
18.2.3 CONSUMABLES
18.2.3.1. NEEDLES AND SYRINGES
18.2.3.2. BLOOD COLLECTION DEVICES
18.2.3.3. MEDIA CULTURE
18.2.3.3.1. BROTH
18.2.3.3.2. AGAR
18.2.3.3.3. OTHERS
18.2.3.4. STAINS
18.2.3.4.1. GRAM STAINS
18.2.3.4.2. MYCOBACTERIA STAINS
18.2.3.4.3. FUNGAL STAINS
18.2.3.4.4. OTHERS
18.2.3.5. CONTROLS & CALIBRATORS
18.2.3.6. PLATES
18.2.3.7. OTHERS
18.3 SERVICES
19 EUROPE DIAGNOSTICS TESTS MARKET, BY TECHNOLOGY
19.1 OVERVIEW
19.2 PCR-BASED
19.2.1 REAL-TIME PCR
19.2.2 NESTED PCR
19.2.3 MULTIPLEX PCR
19.2.4 QUANTITATIVE PCR
19.2.5 OTHERS
19.3 IMMUNOASSAY-BASED
19.3.1 LATERAL FLOW IMMUNOASSAY
19.3.1.1. SANDWICH ASSAYS
19.3.1.2. COMPETITIVE ASSAYS
19.3.2 PAPER-BASED IMMUNOASSAYS
19.3.3 OTHERS
19.4 CHROMATOGRAPHY-BASED
19.4.1 LC-MS
19.4.2 GC-MS
19.4.3 LC-MS/MS
19.5 SPECTROSCOPY-BASED
19.5.1 MASS SPECTROMETRY
19.5.2 RAMAN SPECTROSCOPY
19.5.3 OPTICAL SPECTROSCOPY
19.5.4 OTHERS
19.6 MICROFLUIDICS
19.6.1 LUCIFERASE IMMUNOPRECIPITATION SYSTEMS (LIPS)
19.6.2 PAPER-BASED MICROFLUDICS
19.7 NEXT GENE SEQUENCING
19.7.1 WHOLE GENOME SEQUENCING
19.7.2 TARGETED RESEQUENCING
19.7.3 WHOLE EXOME SEQUENCING
19.7.4 RNA SEQUENCING
19.7.5 OTHERS
19.8 OTHERS
20 EUROPE DIAGNOSTICS TESTS MARKET, BY MODE OF TESTING
20.1 OVERVIEW
20.2 PRESCRIPTION-BASED TESTING
20.3 OTC TESTING
21 EUROPE DIAGNOSTICS TESTS MARKET, BY APPLICATION
21.1 OVERVIEW
21.2 CARDIOLOGY
21.3 ONCOLOGY
21.4 NEUROLOGY
21.5 ORTHOPEDICS
21.6 GASTROENTROLOGY
21.7 GYNECOLOGY
21.8 ODONTOLOGY
21.9 OTHERS
22 EUROPE DIAGNOSTICS TESTS MARKET, BY SAMPLE TYPE
22.1 BLOOD
22.2 URINE
22.3 SALIVA
22.4 RESPIRATORY SAMPLES
22.5 STOOL
22.6 BIOPSY
22.7 OTHERS
23 EUROPE DIAGNOSTICS TESTS MARKET, BY TESTING SITE
23.1 OVERVIEW
23.2 AT HOME TESTING
23.3 LABROATORIES BASED TESTING
24 EUROPE DIAGNOSTICS TESTS MARKET, BY TESTING TYPE
24.1 OVERVIEW
24.2 BIOCHEMISTRY
24.3 HEMATOLOGY
24.4 MICROBIOLOGY
24.5 HISTOPATHOLOGY
24.6 OTHERS
25 EUROPE DIAGNOSTICS TESTS MARKET, BY AGE
25.1 OVERVIEW
25.2 PEDIATRIC
25.3 ADULT
25.4 GERIARTIC
26 EUROPE DIAGNOSTICS TESTS MARKET, BY END USER
26.1 OVERVIEW
26.2 HOSPITALS
26.2.1 PUBLIC
26.2.2 PRIVATE
26.3 DIAGNOSTIC CENTER
26.4 ACADEMIC LABS & INSTITUTES
26.5 RESEARCH INSTITUTE
26.6 HOMECARE
26.7 BLOOD BANKS
26.8 SPECILATY CLINICS
26.9 AMBULATORY SURGICAL CENTERS (ASCS)
26.1 OTHERS
27 EUROPE DIAGNOSTICS TESTS MARKET, BY DISTRIBUTION CHANNEL
27.1 OVERVIEW
27.2 DIRECT TENDERS
27.3 RETAIL SALES
27.3.1 ONLINE
27.3.2 OFFLINE
27.4 OTHERS
28 EUROPE DIAGNOSTICS TESTS MARKET, COMPANY LANDSCAPE
28.1 COMPANY SHARE ANALYSIS: EUROPE
28.2 MERGERS & ACQUISITIONS
28.3 NEW PRODUCT DEVELOPMENT & APPROVALS
28.4 EXPANSIONS
28.5 REGULATORY CHANGES
28.6 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
29 EUROPE DIAGNOSTICS TESTS MARKET, SWOT AND DBMR ANALYSIS
30 EUROPE DIAGNOSTICS TESTS MARKET, BY COUNTRY
EUROPE DIAGNOSTICS TESTS MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
30.1 EUROPE
30.1.1 GERMANY
30.1.2 FRANCE
30.1.3 U.K.
30.1.4 HUNGARY
30.1.5 LITHUANIA
30.1.6 AUSTRIA
30.1.7 IRELAND
30.1.8 NORWAY
30.1.9 POLAND
30.1.10 ITALY
30.1.11 SPAIN
30.1.12 RUSSIA
30.1.13 TURKEY
30.1.14 NETHERLANDS
30.1.15 SWITZERLAND
30.1.16 REST OF EUROPE
30.2 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
31 EUROPE DIAGNOSTICS TESTS MARKET, COMPANY PROFILE
31.1 ABBOTT
31.1.1 COMPANY OVERVIEW
31.1.2 REVENUE ANALYSIS
31.1.3 GEOGRAPHIC PRESENCE
31.1.4 PRODUCT PORTFOLIO
31.1.5 RECENT DEVELOPMENTS
31.2 BIO-RAD LABORATORIES, INC.
31.2.1 COMPANY OVERVIEW
31.2.2 REVENUE ANALYSIS
31.2.3 GEOGRAPHIC PRESENCE
31.2.4 PRODUCT PORTFOLIO
31.2.5 RECENT DEVELOPMENTS
31.3 DANAHER
31.3.1 COMPANY OVERVIEW
31.3.2 REVENUE ANALYSIS
31.3.3 GEOGRAPHIC PRESENCE
31.3.4 PRODUCT PORTFOLIO
31.3.5 RECENT DEVELOPMENTS
31.4 BD
31.4.1 COMPANY OVERVIEW
31.4.2 REVENUE ANALYSIS
31.4.3 GEOGRAPHIC PRESENCE
31.4.4 PRODUCT PORTFOLIO
31.4.5 RECENT DEVELOPMENTS
31.5 F. HOFFMANN-LA ROCHE LTD
31.5.1 COMPANY OVERVIEW
31.5.2 REVENUE ANALYSIS
31.5.3 GEOGRAPHIC PRESENCE
31.5.4 PRODUCT PORTFOLIO
31.5.5 RECENT DEVELOPMENTS
31.6 EKF DIAGNOSTICS
31.6.1 COMPANY OVERVIEW
31.6.2 REVENUE ANALYSIS
31.6.3 GEOGRAPHIC PRESENCE
31.6.4 PRODUCT PORTFOLIO
31.6.5 RECENT DEVELOPMENTS
31.7 SIEMENS HEALTHINEERS
31.7.1 COMPANY OVERVIEW
31.7.2 REVENUE ANALYSIS
31.7.3 GEOGRAPHIC PRESENCE
31.7.4 PRODUCT PORTFOLIO
31.7.5 RECENT DEVELOPMENTS
31.8 THERMO FISHER SCIENTIFIC INC.
31.8.1 COMPANY OVERVIEW
31.8.2 REVENUE ANALYSIS
31.8.3 GEOGRAPHIC PRESENCE
31.8.4 PRODUCT PORTFOLIO
31.8.5 RECENT DEVELOPMENTS
31.9 ACON LABORATORIES, INC.
31.9.1 COMPANY OVERVIEW
31.9.2 REVENUE ANALYSIS
31.9.3 GEOGRAPHIC PRESENCE
31.9.4 PRODUCT PORTFOLIO
31.9.5 RECENT DEVELOPMENTS
31.1 BIOMEDOMICS INC
31.10.1 COMPANY OVERVIEW
31.10.2 REVENUE ANALYSIS
31.10.3 GEOGRAPHIC PRESENCE
31.10.4 PRODUCT PORTFOLIO
31.10.5 RECENT DEVELOPMENTS
31.11 QUIDELORTHO CORPORATION.
31.11.1 COMPANY OVERVIEW
31.11.2 REVENUE ANALYSIS
31.11.3 GEOGRAPHIC PRESENCE
31.11.4 PRODUCT PORTFOLIO
31.11.5 RECENT DEVELOPMENTS
31.12 ARKRAY, INC.
31.12.1 COMPANY OVERVIEW
31.12.2 REVENUE ANALYSIS
31.12.3 GEOGRAPHIC PRESENCE
31.12.4 PRODUCT PORTFOLIO
31.12.5 RECENT DEVELOPMENTS
31.13 STRECK
31.13.1 COMPANY OVERVIEW
31.13.2 REVENUE ANALYSIS
31.13.3 GEOGRAPHIC PRESENCE
31.13.4 PRODUCT PORTFOLIO
31.13.5 RECENT DEVELOPMENTS
31.14 PERKINELMER
31.14.1 COMPANY OVERVIEW
31.14.2 REVENUE ANALYSIS
31.14.3 GEOGRAPHIC PRESENCE
31.14.4 PRODUCT PORTFOLIO
31.14.5 RECENT DEVELOPMENTS
31.15 DIASORIN S.P.A
31.15.1 COMPANY OVERVIEW
31.15.2 REVENUE ANALYSIS
31.15.3 GEOGRAPHIC PRESENCE
31.15.4 PRODUCT PORTFOLIO
31.15.5 RECENT DEVELOPMENTS
31.16 BIOHIT HEALTHCARE LTD
31.16.1 COMPANY OVERVIEW
31.16.2 REVENUE ANALYSIS
31.16.3 GEOGRAPHIC PRESENCE
31.16.4 PRODUCT PORTFOLIO
31.16.5 RECENT DEVELOPMENTS
31.17 MICROGEN, INC.
31.17.1 COMPANY OVERVIEW
31.17.2 REVENUE ANALYSIS
31.17.3 GEOGRAPHIC PRESENCE
31.17.4 PRODUCT PORTFOLIO
31.17.5 RECENT DEVELOPMENTS
31.18 TRINITY BIOTECH IRELAND
31.18.1 COMPANY OVERVIEW
31.18.2 REVENUE ANALYSIS
31.18.3 GEOGRAPHIC PRESENCE
31.18.4 PRODUCT PORTFOLIO
31.18.5 RECENT DEVELOPMENTS
31.19 SYSMEX EUROPE SE
31.19.1 COMPANY OVERVIEW
31.19.2 REVENUE ANALYSIS
31.19.3 GEOGRAPHIC PRESENCE
31.19.4 PRODUCT PORTFOLIO
31.19.5 RECENT DEVELOPMENTS
31.2 QIAGEN
31.20.1 COMPANY OVERVIEW
31.20.2 REVENUE ANALYSIS
31.20.3 GEOGRAPHIC PRESENCE
31.20.4 PRODUCT PORTFOLIO
31.20.5 RECENT DEVELOPMENTS
31.21 BIOMÉRIEUX SA
31.21.1 COMPANY OVERVIEW
31.21.2 REVENUE ANALYSIS
31.21.3 GEOGRAPHIC PRESENCE
31.21.4 PRODUCT PORTFOLIO
31.21.5 RECENT DEVELOPMENTS
31.22 CHEMBIO DIAGNOSTICS, INC
31.22.1 COMPANY OVERVIEW
31.22.2 REVENUE ANALYSIS
31.22.3 GEOGRAPHIC PRESENCE
31.22.4 PRODUCT PORTFOLIO
31.22.5 RECENT DEVELOPMENTS
31.23 PTS DIAGNOSTICS
31.23.1 COMPANY OVERVIEW
31.23.2 REVENUE ANALYSIS
31.23.3 GEOGRAPHIC PRESENCE
31.23.4 PRODUCT PORTFOLIO
31.23.5 RECENT DEVELOPMENTS
31.24 AGILENT TECHNOLOGIES, INC.
31.24.1 COMPANY OVERVIEW
31.24.2 REVENUE ANALYSIS
31.24.3 GEOGRAPHIC PRESENCE
31.24.4 PRODUCT PORTFOLIO
31.24.5 RECENT DEVELOPMENTS
31.25 ILLUMINA, INC.
31.25.1 COMPANY OVERVIEW
31.25.2 REVENUE ANALYSIS
31.25.3 GEOGRAPHIC PRESENCE
31.25.4 PRODUCT PORTFOLIO
31.25.5 RECENT DEVELOPMENTS
31.26 EXACT SCIENCES CORPORATION
31.26.1 COMPANY OVERVIEW
31.26.2 REVENUE ANALYSIS
31.26.3 GEOGRAPHIC PRESENCE
31.26.4 PRODUCT PORTFOLIO
31.26.5 RECENT DEVELOPMENTS
31.27 MYRIAD GENETICS, INC.
31.27.1 COMPANY OVERVIEW
31.27.2 REVENUE ANALYSIS
31.27.3 GEOGRAPHIC PRESENCE
31.27.4 PRODUCT PORTFOLIO
31.27.5 RECENT DEVELOPMENTS
31.28 QUEST DIAGNOSTICS INCORPORATED.
31.28.1 COMPANY OVERVIEW
31.28.2 REVENUE ANALYSIS
31.28.3 GEOGRAPHIC PRESENCE
31.28.4 PRODUCT PORTFOLIO
31.28.5 RECENT DEVELOPMENTS
31.29 LABORATORY CORPORATION OF AMERICA HOLDINGS.
31.29.1 COMPANY OVERVIEW
31.29.2 REVENUE ANALYSIS
31.29.3 GEOGRAPHIC PRESENCE
31.29.4 PRODUCT PORTFOLIO
31.29.5 RECENT DEVELOPMENTS
31.3 PHC HOLDINGS CORPORATION
31.30.1 COMPANY OVERVIEW
31.30.2 REVENUE ANALYSIS
31.30.3 GEOGRAPHIC PRESENCE
31.30.4 PRODUCT PORTFOLIO
31.30.5 RECENT DEVELOPMENTS
32 RELATED REPORTS
33 CONCLUSION
34 QUESTIONNAIRE
35 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












