Global Cardiovascular Disease Drug Market
Market Size in USD Million
CAGR :
% 
 USD
155.60 Million
USD
230.24 Million
2021
2029
USD
155.60 Million
USD
230.24 Million
2021
2029
| 2022 –2029 | |
| USD 155.60 Million | |
| USD 230.24 Million | |
|
|
|
|
Cardiovascular Disease Drug Market Analysis and Size
The global cardiovascular disease drug market is expected to witness huge growth in the forecast period. Rising cases of hypertension and other cardiovascular diseases boosts the non-cardioselective beta blockers market. It is used to prevent or improve symptoms in people who've abnormal coronary heart rhythm, Chest ache, coronary heart assaults, coronary heart failure, migraine, and kinds of tremors. COVID-19 also had a major impact on the market growth.
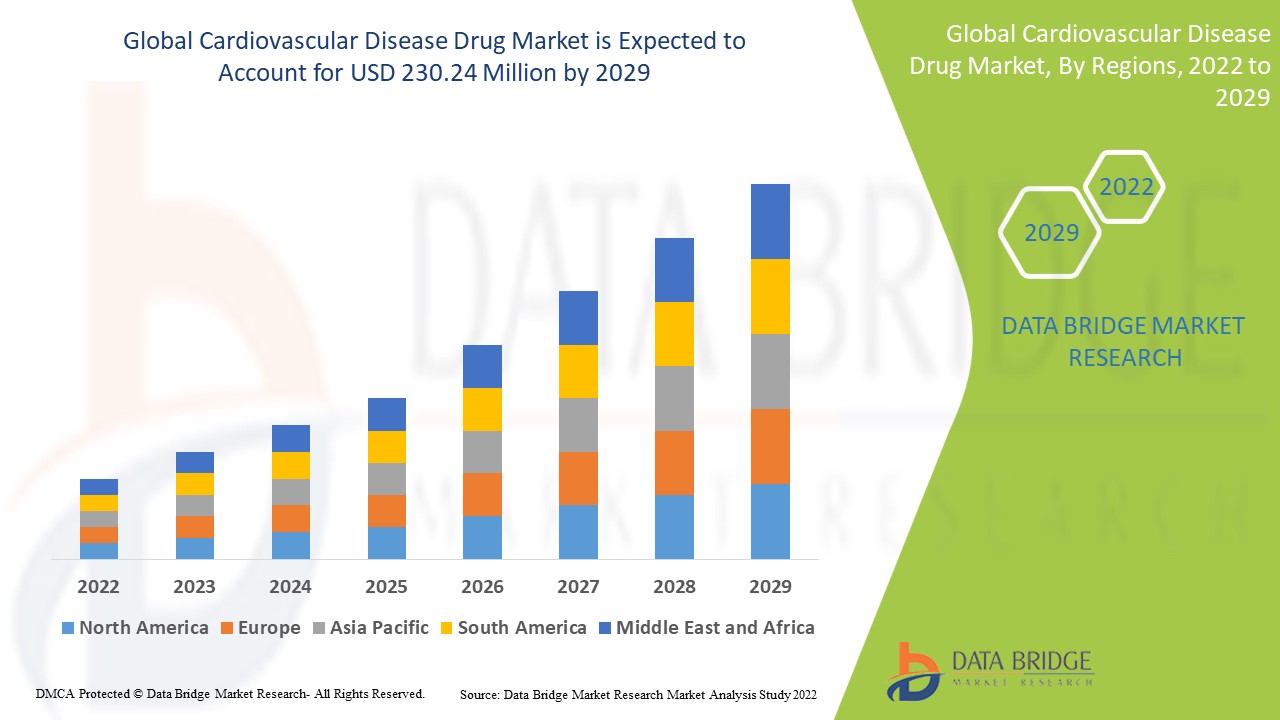
Data Bridge Market Research analyses a growth rate in the global cardiovascular disease drug market in the forecast period 2022-2029. The expected CAGR of global cardiovascular disease drug market is tend to be around 5.02% in the mentioned forecast period. The market was valued at USD 155.6 million in 2021, and it would grow upto USD 230.24 million by 2029. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Cardiovascular Disease Drug Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2014 - 2019) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Diseases (Hypertension, Dyslipidemia, Inflammatory Heart Disease, Ischemic Heart Disease, Others), Treatment (Antiplatelet, Agents, Beta-Blockers, Angiotensin-Converting Enzyme Inhibitors, Others), Route of Administration (Oral, Parenteral, Others), End-Users (Hospitals, Homecare, Specialty Clinics, Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, Retail Pharmacy) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
F. Hoffmann-La Roche Ltd (Switzerland), Fresenius Kabi AG (Germany), Bayer AG (Germany), Sun Pharmaceutical Industries Ltd (India), Novartis AG (Switzerland), Mylan N.V. (U.S.), Teva Pharmaceutical Industries Ltd (Israel), Zydus Group (India), Pfizer Inc (U.S.), Lupin (India), GSK Plc (U.K.), Glenmark Pharmaceuticals Inc (India), Capricor Therapeutics (U.S.), Zensun (China) |
|
Market Opportunities |
|
Market Definition
The disorders or conditions affecting the structures and function of the heart and blood vessels are known as cardiovascular diseases. Cardiovascular disease is recognized as one of the major top cause of global death. It is generally a prolonged condition and affects mainly the elderly population. Cardiovascular drugs cannot be discontinued easily by a patient as it requires a proper judgment of the patient's cardiac health that may prove to be fatal in the long run.
Global Cardiovascular Disease Drug Market Dynamics
Drivers
- Increase in Cardiovascular Diseases
According to the WHO, around 60% - 85% of the population have adopted a sedentary life. According to CDC, approximately 31 million adults aged over 50 years live a sedentary life, and only 1 out of 4 U.S. adults meet the appropriate need of physical activity. This boost the market growth.
- Rising FDA Approvals
The persistent product approvals are expected to drive the market growth. For instance, in May 2022, Zydus Lifesciences Limited’s subsidiary, received tentative approval from the U.S. FDA to market Selexipag tablets that is used to treat pulmonary arterial hypertension (PAH) in adults. In addition, in February 2022, the FDA approved the Norliqva (amlodipine) oral solution to treat hypertension in adults and children 6 years or older, to lower blood pressure and coronary artery disease.
Opportunities
- Rising Prevalence of Hypertension
As per the American College of Cardiology, older adults are mostly undertreated for high blood pressure, despite having the maximum prevalence of hypertension and the highest risk of CV morbidity and mortality (BP). According to the National Health and Nutrition Examination Survey (NHANES) records in the U.S., hypertension affects 70% of persons over 65 years. As our population ages, this figure will continue to rise. In 2014, 15% of the US population was 65 years old, and that number is projected to rise to 20% by 2050. This will create more opportunities for the market growth as the demand for blockers also increases.
- Increasing Demand for Retail Pharmacies
The rise in the number of cardiovascular disease drug delivered through retail pharmacies and the rise in the number of retail pharmacies in highly developed countries can create opportunities for the market growth. Additionally, patients prefer retail pharmacies for purchasing drugs, as these are easily accessible.
Restraints/Challenges
- Lack of skilled professionals
The lack of qualified healthcare professionals who cannot treat the patients with these agents could impede the growth of the global cardiovascular disease drug market over a forecast period.
- Side Effects of Cardioselective Beta Blockers
There are various side effects associated with cardiovascular disease drug such as upset stomach, nausea, diarrhea or constipation, and erectile dysfunction when taking beta-blockers that hamper the market growth.
This global cardiovascular disease drug market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the global cardioselective beta blockers market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on Global Cardiovascular Disease Drug Market
The COVID-19 pandemic faced moderately great impact on the market growth. Hypertension is one of the most common phenomenon in COVID-19 patients; thus, beta-blockers are widely used. In addition to this, the pandemic is interrupting the medical supply chain, and the delivery of the prooducts. The COVID-19 pandemic affected healthcare systems worldwide and interrupted usual care in many healthcare facilities, exposing vulnerable patients with cardiovascular diseases to significant risks. Though, the demand for cardiovascular drugs increased during the pandemic due to the increased risk of infection among patients with cardiovascular diseases (CVDs). But in the post pandemic era, the market is rising as the hospitals and clinics are free from the tedious responsibilities of COVID-19 patients.Thus, the market will undergo growth during the forecast period.
Recent Developments:
- In May 2022, Amgen revealed positive data from the Phase 2 OCEAN(a)-DOSE clinical study, evaluating olpasiran in 281 adult patients with Lipoprotein(a), or Lp(a), levels over 150 nmol/L and evidence of atherosclerotic cardiovascular disease (ASCVD). Olpasiran is a small interfering RNA designed to lower the body's production of apolipoprotein(a), a key component of Lp(a) associated with an increased risk of cardiovascular events.
Global Cardiovascular Disease Drug Market Scope
The global cardiovascular disease drug market is segmented on the basis of diseases, treatment, route of administration, distribution channel and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Diseases
- Hypertension
- Dyslipidemia
- Inflammatory Heart Disease
- Ischemic Heart Disease
- Others
Treatment
- Antiplatelet, Agents
- Beta-Blockers
- Angiotensin-Converting Enzyme Inhibitors
- Others
Route of Administration
- Oral
- Parenteral
- Others
End User
- Hospitals
- Homecare
- Specialty Clinics
- Others
Distribution Channel
- Hospital Pharmacy
- Online Pharmacy
- Retail Pharmacy
Cardiovascular Disease Drug Market Regional Analysis/Insights
The global cardiovascular disease drug market is analyzed and market size insights and trends are provided by diseases, treatment, route of administration, distribution channel and end-user as referenced above.
The major countries covered in the global cardiovascular disease drug market report are the U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America has been witnessing a positive growth for global cardiovascular disease drug market throughout the forecasted period due to the global leaders in research and development activities, high incidence of heart diseases and presence of refined medical facilities.
Asia-Pacific dominates the market due to the developing healthcare facilities, large number of generic manufacturer and rise in government initiatives and specialist communities.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Global Cardiovascular Disease Drug Market Share Analysis
The global cardiovascular disease drug market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to global cardiovascular disease drug market
Key players operating in the global cardiovascular disease drug market include:
- F. Hoffmann-La Roche Ltd (Switzerland)
- Fresenius Kabi AG (Germany)
- Bayer AG (Germany)
- Sun Pharmaceutical Industries Ltd (India)
- Novartis AG (Switzerland)
- Mylan N.V. (U.S.)
- Teva Pharmaceutical Industries Ltd (Israel)
- Zydus Group (India)
- Pfizer Inc (U.S.)
- Lupin (India)
- GSK Plc (U.K.)
- Glenmark Pharmaceuticals Inc (India)
- Capricor Therapeutics (U.S.)
- Zensun (China)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL CARDIOVASCULAR DISEASE DRUG MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL CARDIOVASCULAR DISEASE DRUG MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL CARDIOVASCULAR DISEASE DRUG MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
6 EPIDEMIOLOGY
6.1 INCIDENCE OF ALL BY GENDER
6.2 TREATMENT RATE
6.3 MORTALITY RATE
6.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
6.5 PATEINT TREATMENT SUCCESS RATES
7 INDUSTRY INSIGHTS
7.1 PATENT ANALYSIS
7.2 DRUG TREATMENT RATE BY MATURED MARKETS
7.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
7.4 PATIENT FLOW DIAGRAM
7.5 KEY PRICING STRATEGIES
7.6 KEY PATIENT ENROLLMENT STRATEGIES
7.7 INTERVIEWS WITH CARDIOLOGIST
7.8 OTHER KOL SNAPSHOTS
8 REGULATORY SCENARIO
9 PIPELINE ANALYSIS
9.1 PHASE III CANDIDATES
9.2 PHASE II CANDIDATES
9.3 PHASE I CANDIDATES
9.4 OTHERS (PRE-CLINICAL AND RESEARCH)
10 GLOBAL CARDIOVASCULAR DISEASE DRUG MARKET, BY TYPE
10.1 OVERVIEW
10.2 ACE INHIBITORS
10.2.1 BENAZEPRIL
10.2.2 CAPTOPRIL
10.2.3 ENALAPRIL MALEATE
10.2.4 LISINOPRIL
10.2.5 OTHERS
10.3 ANGIOTENSIN II RECEPTOR ANTAGONISTS (ARBS)
10.3.1 CANDESARTAN CILEXETIL
10.3.2 EPROSARTAN MESYLATE
10.3.3 IRBESARTAN
10.3.4 LOSARTAN
10.3.5 OTHERS
10.4 ANTIARRHYTHMICS
10.4.1 AMIODARONE
10.4.2 DISOPYRAMIDE PHOSPHATE
10.4.3 DOFETILIDE
10.4.4 FLECAINIDE
10.4.5 MEXILETINE HCL
10.4.6 PROCAINAMIDE
10.4.7 OTHERS
10.5 ANTICOAGULANTS
10.5.1 NON-VKA ORAL ANTICOAGULANTS (NOACS)
10.5.1.1. RIVAROXABAN
10.5.1.2. EDOXABAN
10.5.1.3. APIXABAN
10.5.1.4. OTHERS
10.5.2 HEPARIN & LMWH
10.5.2.1. DALTEPARIN
10.5.2.2. ENOXAPARIN
10.5.2.3. TINZAPARIN
10.5.2.4. OTHERS
10.5.3 VITAMIN K ANTAGONIST
10.5.3.1. WARFARIN
10.5.3.2. PHENPROCOUMON
10.5.3.3. OTHERS
10.5.4 THROMBIN INHIBITORS
10.5.4.1. BIVALIRUDIN
10.5.4.2. ARGATROBAN
10.5.4.3. DABIGATRAN
10.5.4.4. OTHERS
10.5.5 OTHERS
10.6 PLATELET INHIBITORS
10.6.1 ASPIRIN
10.6.2 CILOSTAZOL
10.6.3 CLOPIDOGRIL BISULFATE
10.6.4 DIPYRAMIDAMOLE
10.6.5 OTHERS
10.7 ANTIHYPERTENSIVES
10.7.1 CLONIDINE HCL
10.7.2 DOXAZOSIN MESYLATE
10.7.3 HYDRALAZINE HCI
10.7.4 METHYLDOPA
10.7.5 MINOXIDIL
10.7.6 OTHERS
10.8 BETA BLOCKERS
10.8.1 ACEBUTOLOL HCL
10.8.2 ATENOLOL
10.8.3 BETAXOLOL
10.8.4 BISOPROLOL
10.8.5 CARVEDILOL
10.8.6 LABETALOL HCL
10.8.7 METOPROLOL
10.8.8 METOPROLOL
10.8.9 NADOLOL
10.8.10 OTHERS
10.9 CALCIUM CHANNEL BLOCKERS
10.9.1 DIHYDROPYRIDINES
10.9.1.1. AMLODIPINE BESYLATE
10.9.1.2. NIFEDIPINE
10.9.1.3. NIMODIPINE
10.9.1.4. NISOLDIPINE
10.9.1.5. NICARDIPINE HCL
10.9.2 NONDIHYDROPYRIDINES
10.9.2.1. DILTIAZEM HCL
10.9.2.2. VERAPAMIL HCL
10.1 DIURETICS
10.10.1 THIAZIDE DIURETICS
10.10.1.1. CHLORTHALIDONE
10.10.1.2. HYDROCHLOROTHIAZIDE
10.10.1.3. METOLAZONE
10.10.1.4. INDAPAMIDE
10.10.2 LOOP DIURETICS
10.10.2.1. TORSEMIDE
10.10.2.2. FUROSEMIDE
10.10.2.3. BUMETANIDE
10.10.3 POTASSIUM-SPARING DIURETICS
10.10.3.1. AMILORIDE
10.10.3.2. TRIAMTERENE
10.10.3.3. SPIRONOLACTONE
10.10.3.4. EPLERENONE
10.10.4 OTHERS
10.11 LIPID MEDICATIONS
10.11.1 STATINS
10.11.1.1. ATORVASTATIN CALCIUM
10.11.1.2. FLUVASTATIN SODIUM
10.11.1.3. LOVASTATIN
10.11.1.4. OTHERS
10.11.2 FIBRATES
10.11.2.1. FENOFIBRATE
10.11.2.2. GEMFIBROZIL
10.11.3 BILE ACID SEQUESTRANTS
10.11.3.1. COLESEVELAM HCL
10.11.3.2. CHOLESTYRAMINE
10.11.3.3. COLESTIPOL HCL
10.11.4 OTHER LIPID MEDICATIONS
10.12 NITRATES
10.12.1 ORAL NITROGLYCERIN
10.12.2 NITROGLYCERIN OINTMENT
10.12.3 NITROGLYCERIN SKIN PATCHES
10.12.4 NITROGLYCERIN SUBLINGUAL TABLETS
10.12.5 OTHER NITROGLYCERIN TABLETS, CAPSULES, AND SPRAYS
10.13 OTHERS
11 GLOBAL CARDIOVASCULAR DISEASE DRUG MARKET, BY THERAPEUTIC AREAS
11.1 OVERVIEW
11.2 CORONARY HEART DISEASE
11.2.1 ACE INHIBITORS
11.2.2 ANGIOTENSIN II RECEPTOR ANTAGONISTS (ARBS)
11.2.3 ANTIARRHYTHMICS
11.2.4 ANTICOAGULANTS
11.2.5 PLATELET INHIBITORS
11.2.6 ANTIHYPERTENSIVES
11.2.7 BETA BLOCKERS
11.2.8 CALCIUM CHANNEL BLOCKERS
11.2.9 DIURETICS
11.2.10 LIPID MEDICATIONS
11.2.11 NITRATES
11.2.12 OTHERS
11.3 CEREBROVASCULAR DISEASE
11.3.1 ACE INHIBITORS
11.3.2 ANGIOTENSIN II RECEPTOR ANTAGONISTS (ARBS)
11.3.3 ANTIARRHYTHMICS
11.3.4 ANTICOAGULANTS
11.3.5 PLATELET INHIBITORS
11.3.6 ANTIHYPERTENSIVES
11.3.7 BETA BLOCKERS
11.3.8 CALCIUM CHANNEL BLOCKERS
11.3.9 DIURETICS
11.3.10 LIPID MEDICATIONS
11.3.11 NITRATES
11.3.12 OTHERS
11.4 PERIPHERAL ARTERIAL DISEASE
11.4.1 ACE INHIBITORS
11.4.2 ANGIOTENSIN II RECEPTOR ANTAGONISTS (ARBS)
11.4.3 ANTIARRHYTHMICS
11.4.4 ANTICOAGULANTS
11.4.5 PLATELET INHIBITORS
11.4.6 ANTIHYPERTENSIVES
11.4.7 BETA BLOCKERS
11.4.8 CALCIUM CHANNEL BLOCKERS
11.4.9 DIURETICS
11.4.10 LIPID MEDICATIONS
11.4.11 NITRATES
11.4.12 OTHERS
11.5 RHEUMATIC HEART DISEASE
11.5.1 ACE INHIBITORS
11.5.2 ANGIOTENSIN II RECEPTOR ANTAGONISTS (ARBS)
11.5.3 ANTIARRHYTHMICS
11.5.4 ANTICOAGULANTS
11.5.5 PLATELET INHIBITORS
11.5.6 ANTIHYPERTENSIVES
11.5.7 BETA BLOCKERS
11.5.8 CALCIUM CHANNEL BLOCKERS
11.5.9 DIURETICS
11.5.10 LIPID MEDICATIONS
11.5.11 NITRATES
11.5.12 OTHERS
11.6 CONGENITAL HEART DISEASE
11.6.1 ACE INHIBITORS
11.6.2 ANGIOTENSIN II RECEPTOR ANTAGONISTS (ARBS)
11.6.3 ANTIARRHYTHMICS
11.6.4 ANTICOAGULANTS
11.6.5 PLATELET INHIBITORS
11.6.6 ANTIHYPERTENSIVES
11.6.7 BETA BLOCKERS
11.6.8 CALCIUM CHANNEL BLOCKERS
11.6.9 DIURETICS
11.6.10 LIPID MEDICATIONS
11.6.11 NITRATES
11.6.12 OTHERS
11.7 DEEP VEIN THROMBOSIS AND PULMONARY EMBOLISM
11.7.1 ACE INHIBITORS
11.7.2 ANGIOTENSIN II RECEPTOR ANTAGONISTS (ARBS)
11.7.3 ANTIARRHYTHMICS
11.7.4 ANTICOAGULANTS
11.7.5 PLATELET INHIBITORS
11.7.6 ANTIHYPERTENSIVES
11.7.7 BETA BLOCKERS
11.7.8 CALCIUM CHANNEL BLOCKERS
11.7.9 DIURETICS
11.7.10 LIPID MEDICATIONS
11.7.11 NITRATES
11.7.12 OTHERS
11.8 OTHERS
12 GLOBAL CARDIOVASCULAR DISEASE DRUG MARKET, BY ROUTE OF ADMINISTRATION
12.1 OVERVIEW
12.2 ORAL
12.2.1 SOLID
12.2.1.1. TABLETS
12.2.1.2. CAPSULES
12.2.1.3. OTHERS
12.2.2 LIQUID
12.2.2.1. EMULSIONS
12.2.2.2. ELIXIRS
12.2.2.3. SOLUTIONS
12.2.2.4. SYRUPS
12.2.2.5. SUSPENSIONS
12.2.2.6. OTHERS
12.3 PARENTERAL
12.3.1 CONVENTIONAL DRUG DELIVERY FORMULATIONS
12.3.1.1. SOLUTIONS
12.3.1.2. RECONSTITUTED/LYOPHILIZED
12.3.1.3. SUSPENSIONS
12.3.1.4. EMULSIONS
12.3.1.5. OTHERS
12.3.2 NOVEL DRUG DELIVERY FORMULATIONS
12.3.2.1. COLLOIDAL DISPERSIONS
12.3.2.2. LONG ACTING INJECTION FORMULATION
13 GLOBAL CARDIOVASCULAR DISEASE DRUG MARKET, BY DRUG TYPE
13.1 OVERVIEW
13.2 PRESCRIPTION
13.3 OVER THE COUNTER (OTC)
14 GLOBAL CARDIOVASCULAR DISEASE DRUG MARKET, BY PRODUCT TYPE
14.1 OVERVIEW
14.2 BRANDED
14.3 GENERICS
15 GLOBAL CARDIOVASCULAR DISEASE DRUG MARKET, BY END USER
15.1 OVERVIEW
15.2 HOSPITAL
15.3 CLINICS
15.4 HOME HEALTHCARE
15.5 OTHERS
16 GLOBAL CARDIOVASCULAR DISEASE DRUG MARKET, BY DISTRIBUTION CHANNEL
16.1 OVERVIEW
16.2 HOSPITAL PHARMACY
16.3 RETAIL PHARMACY
16.4 ONLINE PHARMACY
16.5 OTHERS
17 GLOBAL CARDIOVASCULAR DISEASE DRUG MARKET, BY GEOGRAPHY
GLOBAL CARDIOVASCULAR DISEASE DRUG MARKET (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
17.1 NORTH AMERICA
17.1.1 U.S.
17.1.1.1. U.S. CARDIOVASCULAR DISEASE DRUG MARKET, BY TYPE
17.1.1.2. U.S. CARDIOVASCULAR DISEASE DRUG MARKET, BY THERAPEUTIC AREAS
17.1.1.3. U.S. CARDIOVASCULAR DISEASE DRUG MARKET, BY ROUTE OF ADMINISTRATION
17.1.1.4. U.S. CARDIOVASCULAR DISEASE DRUG MARKET, BY DRUG TYPE
17.1.1.5. U.S. CARDIOVASCULAR DISEASE DRUG MARKET, BY PRODUCT TYPE
17.1.1.6. U.S. CARDIOVASCULAR DISEASE DRUG MARKET, BY END USER
17.1.1.7. U.S. CARDIOVASCULAR DISEASE DRUG MARKET, BY DISTRIBUTION CHANNEL
17.1.2 CANADA
17.1.3 MEXICO
17.2 EUROPE
17.2.1 GERMANY
17.2.2 FRANCE
17.2.3 U.K.
17.2.4 HUNGARY
17.2.5 LITHUANIA
17.2.6 AUSTRIA
17.2.7 IRELAND
17.2.8 NORWAY
17.2.9 POLAND
17.2.10 ITALY
17.2.11 SPAIN
17.2.12 RUSSIA
17.2.13 TURKEY
17.2.14 NETHERLANDS
17.2.15 SWITZERLAND
17.2.16 REST OF EUROPE
17.3 ASIA-PACIFIC
17.3.1 JAPAN
17.3.2 CHINA
17.3.3 SOUTH KOREA
17.3.4 INDIA
17.3.5 AUSTRALIA
17.3.6 SINGAPORE
17.3.7 THAILAND
17.3.8 MALAYSIA
17.3.9 INDONESIA
17.3.10 PHILIPPINES
17.3.11 VIETNAM
17.3.12 REST OF ASIA-PACIFIC
17.4 SOUTH AMERICA
17.4.1 BRAZIL
17.4.2 ARGENTINA
17.4.3 PERU
17.4.4 REST OF SOUTH AMERICA
17.5 MIDDLE EAST AND AFRICA
17.5.1 SOUTH AFRICA
17.5.2 SAUDI ARABIA
17.5.3 UAE
17.5.4 EGYPT
17.5.5 KUWAIT
17.5.6 ISRAEL
17.5.7 REST OF MIDDLE EAST AND AFRICA
17.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
18 GLOBAL CARDIOVASCULAR DISEASE DRUG MARKET, SWOT AND DBMR ANALYSIS
19 GLOBAL CARDIOVASCULAR DISEASE DRUG MARKET, COMPANY LANDSCAPE
19.1 COMPANY SHARE ANALYSIS: GLOBAL
19.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
19.3 COMPANY SHARE ANALYSIS: EUROPE
19.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
19.5 MERGERS & ACQUISITIONS
19.6 NEW PRODUCT DEVELOPMENT & APPROVALS
19.7 EXPANSIONS
19.8 REGULATORY CHANGES
19.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
20 GLOBAL CARDIOVASCULAR DISEASE DRUG MARKET, COMPANY PROFILE
20.1 ASTRAZENECA
20.1.1 COMPANY OVERVIEW
20.1.2 REVENUE ANALYSIS
20.1.3 GEOGRAPHIC PRESENCE
20.1.4 PRODUCT PORTFOLIO
20.1.5 RECENT DEVELOPMENTS
20.2 BRISTOL-MYERS SQUIBB
20.2.1 COMPANY OVERVIEW
20.2.2 REVENUE ANALYSIS
20.2.3 GEOGRAPHIC PRESENCE
20.2.4 PRODUCT PORTFOLIO
20.2.5 RECENT DEVELOPMENTS
20.3 NOVARTIS AG
20.3.1 COMPANY OVERVIEW
20.3.2 REVENUE ANALYSIS
20.3.3 GEOGRAPHIC PRESENCE
20.3.4 PRODUCT PORTFOLIO
20.3.5 RECENT DEVELOPMENTS
20.4 PFIZER INC.
20.4.1 COMPANY OVERVIEW
20.4.2 REVENUE ANALYSIS
20.4.3 GEOGRAPHIC PRESENCE
20.4.4 PRODUCT PORTFOLIO
20.4.5 RECENT DEVELOPMENTS
20.5 SANOFI
20.5.1 COMPANY OVERVIEW
20.5.2 REVENUE ANALYSIS
20.5.3 GEOGRAPHIC PRESENCE
20.5.4 PRODUCT PORTFOLIO
20.5.5 RECENT DEVELOPMENTS
20.6 BAYER AG
20.6.1 COMPANY OVERVIEW
20.6.2 REVENUE ANALYSIS
20.6.3 GEOGRAPHIC PRESENCE
20.6.4 PRODUCT PORTFOLIO
20.6.5 RECENT DEVELOPMENTS
20.7 DAIICHI SANKYO CO LTD
20.7.1 COMPANY OVERVIEW
20.7.2 REVENUE ANALYSIS
20.7.3 GEOGRAPHIC PRESENCE
20.7.4 PRODUCT PORTFOLIO
20.7.5 RECENT DEVELOPMENTS
20.8 JOHNSON & JOHNSON SERVICES, INC.
20.8.1 COMPANY OVERVIEW
20.8.2 REVENUE ANALYSIS
20.8.3 GEOGRAPHIC PRESENCE
20.8.4 PRODUCT PORTFOLIO
20.8.5 RECENT DEVELOPMENTS
20.9 MEDOPHARM
20.9.1 COMPANY OVERVIEW
20.9.2 REVENUE ANALYSIS
20.9.3 GEOGRAPHIC PRESENCE
20.9.4 PRODUCT PORTFOLIO
20.9.5 RECENT DEVELOPMENTS
20.1 MERCK HEALTHCARE KGAA
20.10.1 COMPANY OVERVIEW
20.10.2 REVENUE ANALYSIS
20.10.3 GEOGRAPHIC PRESENCE
20.10.4 PRODUCT PORTFOLIO
20.10.5 RECENT DEVELOPMENTS
20.11 ABBVIE INC.
20.11.1 COMPANY OVERVIEW
20.11.2 REVENUE ANALYSIS
20.11.3 GEOGRAPHIC PRESENCE
20.11.4 PRODUCT PORTFOLIO
20.11.5 RECENT DEVELOPMENTS
20.12 GILEAD SCIENCES, INC.
20.12.1 COMPANY OVERVIEW
20.12.2 REVENUE ANALYSIS
20.12.3 GEOGRAPHIC PRESENCE
20.12.4 PRODUCT PORTFOLIO
20.12.5 RECENT DEVELOPMENTS
20.13 UNITED THERAPEUTICS CORPORATION
20.13.1 COMPANY OVERVIEW
20.13.2 REVENUE ANALYSIS
20.13.3 GEOGRAPHIC PRESENCE
20.13.4 PRODUCT PORTFOLIO
20.13.5 RECENT DEVELOPMENTS
20.14 GLAXOSMITHKLINE PLC
20.14.1 COMPANY OVERVIEW
20.14.2 REVENUE ANALYSIS
20.14.3 GEOGRAPHIC PRESENCE
20.14.4 PRODUCT PORTFOLIO
20.14.5 RECENT DEVELOPMENTS
20.15 TAKEDA PHARMACEUTICAL COMPANY LIMITED
20.15.1 COMPANY OVERVIEW
20.15.2 REVENUE ANALYSIS
20.15.3 GEOGRAPHIC PRESENCE
20.15.4 PRODUCT PORTFOLIO
20.15.5 RECENT DEVELOPMENTS
20.16 BOEHRINGER INGELHEIM INTERNATIONAL GMBH
20.16.1 COMPANY OVERVIEW
20.16.2 REVENUE ANALYSIS
20.16.3 GEOGRAPHIC PRESENCE
20.16.4 PRODUCT PORTFOLIO
20.16.5 RECENT DEVELOPMENTS
20.17 HIKMA PHARMACEUTICALS PLC
20.17.1 COMPANY OVERVIEW
20.17.2 REVENUE ANALYSIS
20.17.3 GEOGRAPHIC PRESENCE
20.17.4 PRODUCT PORTFOLIO
20.17.5 RECENT DEVELOPMENTS
20.18 VIATRIS INC.
20.18.1 COMPANY OVERVIEW
20.18.2 REVENUE ANALYSIS
20.18.3 GEOGRAPHIC PRESENCE
20.18.4 PRODUCT PORTFOLIO
20.18.5 RECENT DEVELOPMENTS
20.19 AUROBINDO PHARMA
20.19.1 COMPANY OVERVIEW
20.19.2 REVENUE ANALYSIS
20.19.3 GEOGRAPHIC PRESENCE
20.19.4 PRODUCT PORTFOLIO
20.19.5 RECENT DEVELOPMENTS
20.2 AMNEAL PHARMACEUTICALS LLC
20.20.1 COMPANY OVERVIEW
20.20.2 REVENUE ANALYSIS
20.20.3 GEOGRAPHIC PRESENCE
20.20.4 PRODUCT PORTFOLIO
20.20.5 RECENT DEVELOPMENTS
20.21 AMGEN INC.
20.21.1 COMPANY OVERVIEW
20.21.2 REVENUE ANALYSIS
20.21.3 GEOGRAPHIC PRESENCE
20.21.4 PRODUCT PORTFOLIO
20.21.5 RECENT DEVELOPMENTS
20.22 F. HOFFMANN-LA ROCHE LTD
20.22.1 COMPANY OVERVIEW
20.22.2 REVENUE ANALYSIS
20.22.3 GEOGRAPHIC PRESENCE
20.22.4 PRODUCT PORTFOLIO
20.22.5 RECENT DEVELOPMENTS
20.23 ELI LILLY AND COMPANY
20.23.1 COMPANY OVERVIEW
20.23.2 REVENUE ANALYSIS
20.23.3 GEOGRAPHIC PRESENCE
20.23.4 PRODUCT PORTFOLIO
20.23.5 RECENT DEVELOPMENTS
20.24 AZURITY PHARMACEUTICALS, INC.
20.24.1 COMPANY OVERVIEW
20.24.2 REVENUE ANALYSIS
20.24.3 GEOGRAPHIC PRESENCE
20.24.4 PRODUCT PORTFOLIO
20.24.5 RECENT DEVELOPMENTS
20.25 ZYDUS GROUP
20.25.1 COMPANY OVERVIEW
20.25.2 REVENUE ANALYSIS
20.25.3 GEOGRAPHIC PRESENCE
20.25.4 PRODUCT PORTFOLIO
20.25.5 RECENT DEVELOPMENTS
20.26 LUPIN
20.26.1 COMPANY OVERVIEW
20.26.2 REVENUE ANALYSIS
20.26.3 GEOGRAPHIC PRESENCE
20.26.4 PRODUCT PORTFOLIO
20.26.5 RECENT DEVELOPMENTS
*NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
21 RELATED REPORTS
22 CONCLUSION
23 QUESTIONNAIRE
24 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












