Global Clinical Laboratory Tests Market
Market Size in USD Billion
CAGR :
% 
 USD
206,645.00 Billion
USD
399,819.07 Billion
2021
2029
USD
206,645.00 Billion
USD
399,819.07 Billion
2021
2029
| 2022 –2029 | |
| USD 206,645.00 Billion | |
| USD 399,819.07 Billion | |
|
|
|
|
Clinical Laboratory Tests Market Analysis and Size
According to World Health Organization data, more than 17 million people die each year from cardiovascular disorders caused by a variety of unhealthy behaviours, high blood pressure, and high cholesterol levels. Liver cirrhosis, hepatitis, liver cancer, bone disease, bile duct obstruction, and autoimmune disorders are all part of the liver panel segment. Hepatitis had the highest share in 2019, while liver cirrhosis was expected to be the fastest-growing segment over the forecast period due to rising lifestyle disorders and tobacco and alcohol consumption.
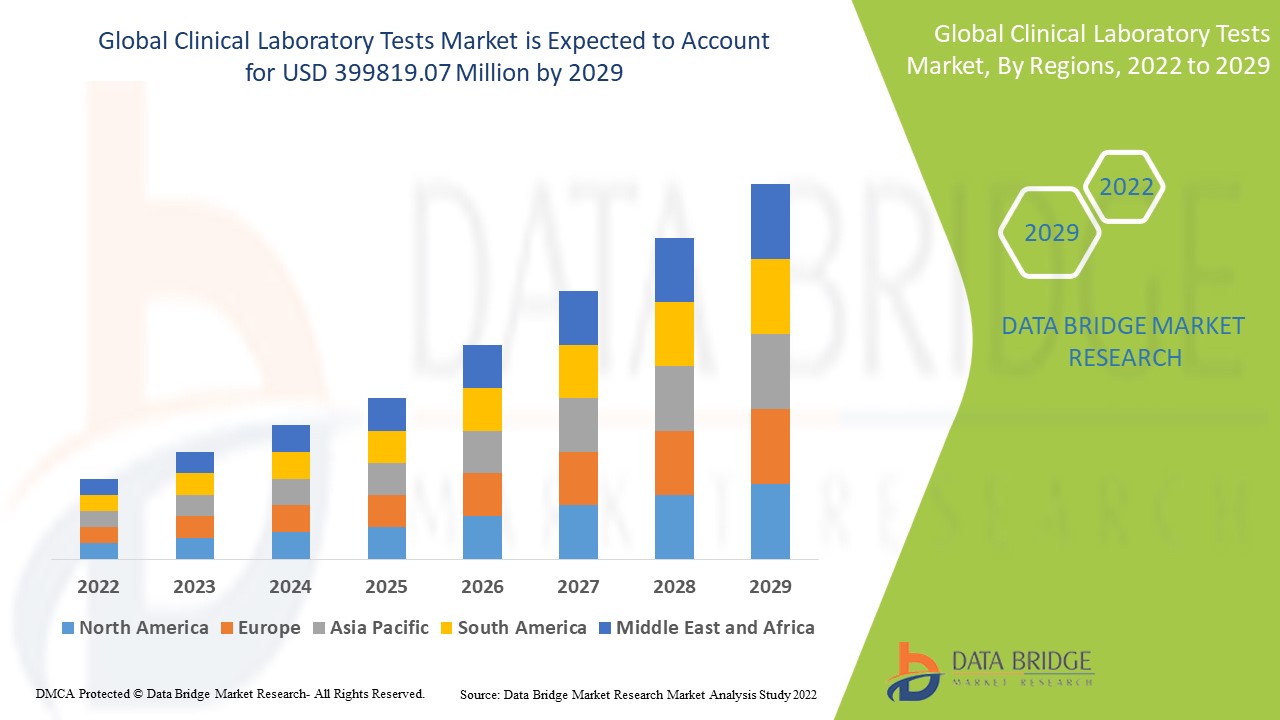
Data Bridge Market Research analyses that the clinical laboratory tests market which was USD 206,645.00 million in 2021, is expected to reach USD 399819.07 million by 2029, at a CAGR of 8.6% during the forecast period 2022 to 2029. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Clinical Laboratory Tests Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2014 - 2019) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Type of Test, Clinical (Complete Comprehensive Test or Complete Body Test), CBC (Complete Body Count), Basic Metabolic Panel (BMP), HGB/HCT), Tests, HbA1c Tests, BUN Creatinine Tests, Electrolytes Tests, Renal Panel Tests, Lipid Panel Tests, Routine, Speciality), Application (Parasitology, Haematology, Virology, Toxicology, Immunology/Serology, Histopathology and Urinalysis, End Users (Hospital Based Laboratories, Clinics Based Laboratories, Central/Independent Laboratories, Physician Office-Based Laboratories and Other - Retail Clinic) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South AUmerica. |
|
Market Players Covered |
Abbott (U.S.), ARUP Laboratories (U.S.), OPKO Health, Inc. (U.S.), ioscientia Healthcare GmbH (Germany), Charles River Laboratories (U.S.), NeoGenomics Laboratories (U.S.), Genoptix, Inc. (U.S.), Healthscope (Australia), The Laboratory Glassware Co. (U.S.), Laboratory Corporation of America® Holdings (U.S.), Fresenius Medical Care AG & Co. KGaA (Germany), QIAGEN (Germany), Quest Diagnostics Incorporated (U.S.), Siemens Healthcare Private Limited (Germany), Tulip Diagnostics (P) Ltd. (India), Sonic Healthcare Limited (Australia), Merck KGaA (Germany) |
|
Market Opportunities |
|
Market Definition
Clinical laboratory test results are used in diagnostic decision making as part of clinical medicine. Clinical laboratory tests are classified into three types. The US Food and Drug Administration (FDA) determines the testing categories of tests that have been cleared for clinical use using a scoring system that considers the complexity of the testing, the stability of calibrators, controls, pre-analytical steps required, and the need for result interpretation. The test samples are analysed by a technician or your doctor to see if your results are within the normal range. Clinical laboratories are medical services that offer a wide range of research techniques to help doctors with diagnosis, therapeutic interventions, and patient supervision. These labs are run by medical technologists (clinical laboratory physicists), who are qualified to conduct experiments on collections of biological samples obtained from their patients. The vast majority of clinical labs are housed within or near medical facilities, making them accessible to both doctors and patients. Clinical laboratory classifications show that these facilities can include high-quality laboratory experiments required to meet clinical and public health demands.
Global Clinical Laboratory Tests Market Dynamics
Drivers
- Growing geriatric population will bolster the growth rate
The growing geriatric population is expected to drive the overall clinical laboratory test market. According to the World Population Prospects: the 2019 Revision data, approximately one in every eleven people in the world were over the age of 65 in 2019, and by 2050, it is estimated that approximately one in every six people will be over the age of 65. Clinical laboratory tests are being used more and more to diagnose age-related diseases.
Furthermore, the rising rate of insufficient exercise, unhealthy food consumption, and the resulting rise in cases of obesity are expected to increase the prevalence of various chronic diseases. The growing awareness of the importance of regular body profiling among healthcare professionals and patients around the world is drive up demand for clinical laboratory tests.
- Rising prevalence of target diseases
The market is expected to benefit from the rising prevalence of target diseases such as cardiovascular disease and diabetes during the forecast period. Cardiovascular diseases are the leading cause of death worldwide. Demand for point-of-care lipid testing is expected to be driven by unmet medical needs related to cardiovascular diseases and increased patient awareness. The World Health Organization predicts that cardiovascular diseases will be the leading cause of death and morbidity in 2021. In the last three decades, 17.7 million people have died from cardiovascular diseases, accounting for 31% of all fatalities worldwide.
The rising prevalence of lifestyle-related diseases such as obesity, the global smoking trend, and an unhealthy diet are among the factors contributing to the global rise in the incidence of renal and lipid-related disorders. Furthermore, as the global prevalence of diabetes rises, so does the number of patients who require clinical laboratory tests.
Opportunities
- Innovative solutions in clinical laboratory tests
Innovative solutions for improving efficiency and eliminating errors are expected to be a major rendering driver in this industry. Integrated workflow management systems, database management tools, and patient test records are becoming increasingly important in the healthcare industry, with organisations processing 100 to 150 billion samples per year. The implementation and development of data management and informatics solutions to support smooth operations are expected to drive market growth.
Restraints/Challenges
- Stringent governmental guidelines will restrict the growth rate
The stringent governmental guidelines and regulations on laboratory research are the main factors limiting the market growth. The maintenance of quality of clinical laboratory products is governed by country specific regulatory bodies. These bodies ensure protection from risks caused by flaws in design, technology, packaging, and manufacturing of the equipment or peripherals.
- Ambiguous regulatory framework
The healthcare industry relies heavily on regulatory frameworks established by organisations such as the FDA and EMA in the United States. There are no specific regulatory requirements for the diagnostic business in emerging markets such as China and India. Laboratory-designed tests (LDTs) are tests developed in-house clinical laboratories that provide accurate and timely results. LDT risks could reduce profit margins and cause delays in the marketing of newly designed tests. This factor is impeding the global clinical laboratory tests market's growth.
This clinical laboratory tests market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the clinical laboratory tests market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent developments
- In April 2022, Quest Diagnostics, based in Secaucus, New Jersey, has announced a number of organisational changes and senior leadership appointments to better support the company's two-point business strategy of accelerating growth and driving operational excellence.
- In April 2022, Abbott has updated its digital health app with its chronic pain neurostimulation devices, making it easier for clinicians to monitor how well patients respond to treatment. While using Abbott's Proclaim device range for spinal cord stimulation (SCS) or dorsal root ganglion (DRG) therapy, the Neurosphere myPath app measures and reports patient-perceived pain relief and general well-being.
Global Clinical Laboratory Tests Market Scope
The clinical laboratory tests market is segmented on the basis of test type, application and end users. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Test Type
- Clinical
- Complete Comprehensive Test or Complete Body Test
- Complete Body Count (CBC)
- Basic Metabolic Panel (BMP)
- HGB/HCT
- HbA1c Tests
- BUN Creatinine Tests
- Electrolytes Tests
- Renal Panel Tests
- Lipid Panel Tests
- Hepatitis
- Bile Duct Obstruction
- Liver Cirrhosis
- Liver Cancer
- Bone Disease
- Autoimmune Disorders
- Others
- Routine
- Speciality
Application
- Parasitology
- Haematology
- Virology
- Toxicology
- Immunology/Serology
- Histopathology
- Urinalysis
End Users
- Hospital Based Laboratories
- Clinics Based Laboratories
- Central/Independent Laboratories
- Physician Office-Based Laboratories
- Retail Clinic
- Other
Clinical Laboratory Tests Market Regional Analysis/Insights
The clinical laboratory tests market is analysed and market size insights and trends are provided by country, test type, application and end users as referenced above.
The countries covered in the clinical laboratory tests market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the clinical laboratory tests market owing to the pervasiveness of numerous pharmaceuticals as well as for biotechnology companies in the region. Additionally, rising advancement in technology can be the driver of region market whereas the geriatric population is also increasing which increase the demand for diagnostic techniques.
Asia-Pacific is expected to grow at the highest growth rate in the forecast period of 2022 to 2029 due to the growing research and development sector and rising investment towards the healthcare sector.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed base and New Technology Penetration
The clinical laboratory tests market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for clinical laboratory tests market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the clinical laboratory tests market. The data is available for historic period 2010-2020.
Competitive Landscape and Clinical Laboratory Tests Market Share Analysis
The clinical laboratory tests market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to clinical laboratory tests market.
Some of the major players operating in the clinical laboratory tests market are:
- Abbott (U.S.)
- ARUP Laboratories (U.S.)
- OPKO Health, Inc. (U.S.)
- Bioscientia Healthcare GmbH (Germany)
- Charles River Laboratories (U.S.)
- NeoGenomics Laboratories (U.S.)
- Genoptix, Inc. (U.S.)
- Healthscope (Australia)
- The Laboratory Glassware Co. (U.S.)
- Laboratory Corporation of America® Holdings (U.S.)
- Fresenius Medical Care AG & Co. KGaA (Germany)
- QIAGEN (Germany)
- Quest Diagnostics Incorporated (U.S.)
- Siemens Healthcare Private Limited (Germany)
- Tulip Diagnostics (P) Ltd. (India)
- Sonic Healthcare Limited (Australia)
- Merck KGaA (Germany)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL CLINICAL LABORATORY TESTS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL CLINICAL LABORATORY TESTS MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VOLUME
2.2.11 VENDOR SHARE ANALYSIS
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL CLINICAL LABORATORY TESTS MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTLE ANALYSIS
5.2 PORTER’S FIVE FORCES
6 REGULATORY SCENARIO
7 INDUSTRY INSIGHTS
8 IMPACT OF COVID-19 PANDEMIC ON THE MARKET
8.1 PRICE IMPACT
8.2 IMPACT ON DEMAND
8.3 IMPACT ON SUPPLY CHAIN
8.4 STRATEGIC DECISION FOR MANUFACTURERS
8.5 CONCLUSION
9 GLOBAL CLINICAL LABORATORY TESTS MARKET, BY TEST
9.1 OVERVIEW
9.2 CLINICAL
9.3 COMPLETE COMPREHENSIVE TEST OR COMPLETE BODY TEST
9.4 COMPLETE BODY COUNT (CBC)
9.5 BASIC METABOLIC PANEL (BMP)
9.6 HGB/HCT
9.7 HBA1C TESTS
9.8 BUN CREATININE TESTS
9.9 ELECTROLYTES TESTS
9.1 RENAL PANEL TESTS
9.11 LIPID PANEL TESTS
9.12 HEPATITIS
9.13 BILE DUCT OBSTRUCTION
9.14 LIVER PANNEL TESTS
9.14.1 HEPATITIS
9.14.2 BILE DUCT OBSTRUCTION
9.14.3 LIVER CIRRHOSIS
9.14.4 LIVER CANCER
9.14.5 BONE DISEASE
9.14.6 AUTOIMMUNE DISORDERS
9.14.7 OTHERS
9.15 BONE DISEASE TEST
9.16 AUTOIMMUNE DISORDERS TEST
9.17 OTHERS
10 GLOBAL CLINICAL LABORATORY TESTS MARKET, BY TYPE
10.1 OVERVIEW
10.2 ROUTINE
10.3 SPECIALTY
11 GLOBAL CLINICAL LABORATORY TESTS MARKET, BY APPLICATIONS
11.1 OVERVIEW
11.2 PARASITOLOGY
11.3 HEMATOLOGY
11.4 VIROLOGY
11.5 TOXICOLOGY
11.6 IMMUNOLOGY/SEROLOGY
11.7 URINALYSIS
11.8 OTHERS
12 GLOBAL CLINICAL LABORATORY TESTS MARKET, BY SPECIALTY
12.1 ORGAN FUNCTION TESTS
12.1.1 KIDNEY
12.1.1.1. CREATININE
12.1.1.2. UREA
12.1.1.3. URIC ACID
12.1.1.4. OTHERS
12.1.2 LIVER
12.1.2.1. AST
12.1.2.2. ALT
12.1.2.3. AST & ALT
12.1.2.4. LDH
12.1.2.5. BILIRUBIN
12.1.2.6. OTHERS
12.1.3 PANCREAS
12.1.3.1. AMYLASE
12.1.3.2. LIPASE
12.1.4 CARDIOVASCULAR
12.1.4.1. TOTAL CHOLESTEROL
12.1.4.2. TRIGLYCERIDES
12.1.4.3. HDL-CHOLESTEROL
12.1.4.4. LDL-CHOLESTEROL
12.1.5 OTHERS
12.2 HORMONE LEVEL TESTS
12.2.1 CORTISOL
12.2.2 TESTOSTERONE
12.2.3 FOLLICLE-STIMULATING HORMONE
12.2.4 LUTEINIZING HORMONE (LH)
12.2.5 ESTRADIOL
12.2.6 PROGESTERONE
12.2.7 PROLACTIN
12.2.8 OTHERS
12.3 SCREENING TESTS
12.3.1 COMPLETE BLOOD COUNT
12.3.1.1. HAEMOGLOBIN
12.3.1.2. RBC & HEMATOCRIT (HCT)
12.3.1.3. WBC (WHITE BLOOD CELLS, LEUKOCYTES)
12.3.1.4. PLATELET
12.3.1.5. OTHERS
12.3.2 PAP SMEAR
12.3.3 URINALYSIS
12.3.4 HGB/HCT TESTING
12.3.5 OTHERS
12.4 INFECTIOUS DISEASE TESTS
12.4.1 FLU PANEL TEST
12.4.2 MONONUCLEOSIS
12.4.3 OTHERS
12.5 SEXUALLY TRANSMITTED INFECTION TESTS
12.5.1 CHLAMYDIA
12.5.2 GONORRHEA
12.5.3 HIV
12.5.4 OTHERS
12.6 CANCER TESTS
12.6.1 LIVER CANCER
12.6.1.1. CA 125
12.6.1.2. PSA,
12.6.1.3. AFP
12.6.1.4. CEA
12.6.1.5. OTHERS
12.6.2 PROSTATE CANCER
12.6.3 OVARIAN CANCER
12.6.4 OTHERS
12.7 AUTOIMMUNE TESTS
12.8 OTHERS
13 GLOBAL CLINICAL LABORATORY TESTS MARKET, BY END USERS
13.1 OVERVIEW
13.2 INDEPENDENT & REFERENCE LABORATORIES
13.3 HOSPITAL-BASED LABORATORIES
13.4 CLINIC-BASED LABORATORIES
13.5 CENTRAL/INDEPENDENT LABORATORIES
13.6 PHYSICIAN OFFICE-BASED LABORATORIES
13.7 OTHERS
14 GLOBAL CLINICAL LABORATORY TESTS MARKET, COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: GLOBAL
14.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
14.3 COMPANY SHARE ANALYSIS: EUROPE
14.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
14.5 MERGERS & ACQUISITIONS
14.6 NEW PRODUCT DEVELOPMENT & APPROVALS
14.7 EXPANSIONS
14.8 REGULATORY CHANGES
14.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
15 GLOBAL CLINICAL LABORATORY TESTS MARKET, BY GEOGRAPHY
GLOBAL CLINICAL LABORATORY TESTS MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
15.1 NORTH AMERICA
15.1.1 U.S.
15.1.2 CANADA
15.1.3 MEXICO
15.2 EUROPE
15.2.1 GERMANY
15.2.2 FRANCE
15.2.3 U.K.
15.2.4 HUNGARY
15.2.5 LITHUANIA
15.2.6 AUSTRIA
15.2.7 IRELAND
15.2.8 NORWAY
15.2.9 POLAND
15.2.10 ITALY
15.2.11 SPAIN
15.2.12 RUSSIA
15.2.13 TURKEY
15.2.14 NETHERLANDS
15.2.15 SWITZERLAND
15.2.16 REST OF EUROPE
15.3 ASIA-PACIFIC
15.3.1 JAPAN
15.3.2 CHINA
15.3.3 SOUTH KOREA
15.3.4 INDIA
15.3.5 SINGAPORE
15.3.6 THAILAND
15.3.7 INDONESIA
15.3.8 MALAYSIA
15.3.9 PHILIPPINES
15.3.10 AUSTRALIA
15.3.11 NEW ZEALAND
15.3.12 VIETNAM
15.3.13 TAIWAN
15.3.14 REST OF ASIA-PACIFIC
15.4 SOUTH AMERICA
15.4.1 BRAZIL
15.4.2 ARGENTINA
15.4.3 PERU
15.4.4 REST OF SOUTH AMERICA
15.5 MIDDLE EAST AND AFRICA
15.5.1 SOUTH AFRICA
15.5.2 SAUDI ARABIA
15.5.3 UAE
15.5.4 EGYPT
15.5.5 KUWAIT
15.5.6 ISRAEL
15.5.7 REST OF MIDDLE EAST AND AFRICA
15.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
16 GLOBAL CLINICAL LABORATORY TESTS MARKET, SWOT AND DBMR ANALYSIS
17 GLOBAL CLINICAL LABORATORY TESTS MARKET, COMPANY PROFILE
17.1 ABBOTT
17.1.1 COMPANY OVERVIEW
17.1.2 GEOGRAPHIC PRESENCE
17.1.3 PRODUCT PORTFOLIO
17.1.4 RECENT DEVELOPMENTS
17.2 ARUP LABORATORIES
17.2.1 COMPANY OVERVIEW
17.2.2 GEOGRAPHIC PRESENCE
17.2.3 PRODUCT PORTFOLIO
17.2.4 RECENT DEVELOPMENTS
17.3 OPKO HEALTH, INC.
17.3.1 COMPANY OVERVIEW
17.3.2 GEOGRAPHIC PRESENCE
17.3.3 PRODUCT PORTFOLIO
17.3.4 RECENT DEVELOPMENTS
17.4 BIOSCIENTIA HEALTHCARE GMBH
17.4.1 COMPANY OVERVIEW
17.4.2 GEOGRAPHIC PRESENCE
17.4.3 PRODUCT PORTFOLIO
17.4.4 RECENT DEVELOPMENTS
17.5 CHARLES RIVER LABORATORIES
17.5.1 COMPANY OVERVIEW
17.5.2 GEOGRAPHIC PRESENCE
17.5.3 PRODUCT PORTFOLIO
17.5.4 RECENT DEVELOPMENTS
17.6 NEOGENOMICS LABORATORIES
17.6.1 COMPANY OVERVIEW
17.6.2 GEOGRAPHIC PRESENCE
17.6.3 PRODUCT PORTFOLIO
17.6.4 RECENT DEVELOPMENTS
17.7 GENOPTIX, INC.
17.7.1 COMPANY OVERVIEW
17.7.2 GEOGRAPHIC PRESENCE
17.7.3 PRODUCT PORTFOLIO
17.7.4 RECENT DEVELOPMENTS
17.8 HEALTHSCOPE
17.8.1 COMPANY OVERVIEW
17.8.2 GEOGRAPHIC PRESENCE
17.8.3 PRODUCT PORTFOLIO
17.8.4 RECENT DEVELOPMENTS
17.9 THE LABORATORY GLASSWARE CO.
17.9.1 COMPANY OVERVIEW
17.9.2 GEOGRAPHIC PRESENCE
17.9.3 PRODUCT PORTFOLIO
17.9.4 RECENT DEVELOPMENTS
17.1 LABORATORY CORPORATION OF AMERICA® HOLDINGS
17.10.1 COMPANY OVERVIEW
17.10.2 GEOGRAPHIC PRESENCE
17.10.3 PRODUCT PORTFOLIO
17.10.4 RECENT DEVELOPMENTS
17.11 FRESENIUS MEDICAL CARE AG & CO. KGAA
17.11.1 COMPANY OVERVIEW
17.11.2 GEOGRAPHIC PRESENCE
17.11.3 PRODUCT PORTFOLIO
17.11.4 RECENT DEVELOPMENTS
17.12 QIAGEN
17.12.1 COMPANY OVERVIEW
17.12.2 GEOGRAPHIC PRESENCE
17.12.3 PRODUCT PORTFOLIO
17.12.4 RECENT DEVELOPMENTS
17.13 QUEST DIAGNOSTICS INCORPORATED
17.13.1 COMPANY OVERVIEW
17.13.2 GEOGRAPHIC PRESENCE
17.13.3 PRODUCT PORTFOLIO
17.13.4 RECENT DEVELOPMENTS
17.14 SIEMENS HEALTHCARE PRIVATE LIMITED
17.14.1 COMPANY OVERVIEW
17.14.2 GEOGRAPHIC PRESENCE
17.14.3 PRODUCT PORTFOLIO
17.14.4 RECENT DEVELOPMENTS
17.15 TULIP DIAGNOSTICS (P) LTD.
17.15.1 COMPANY OVERVIEW
17.15.2 GEOGRAPHIC PRESENCE
17.15.3 PRODUCT PORTFOLIO
17.15.4 RECENT DEVELOPMENTS
17.16 SONIC HEALTHCARE LIMITED
17.16.1 COMPANY OVERVIEW
17.16.2 GEOGRAPHIC PRESENCE
17.16.3 PRODUCT PORTFOLIO
17.16.4 RECENT DEVELOPMENTS
17.17 MERCK KGAA
17.17.1 COMPANY OVERVIEW
17.17.2 GEOGRAPHIC PRESENCE
17.17.3 PRODUCT PORTFOLIO
17.17.4 RECENT DEVELOPMENTS
17.18 LABCORP
17.18.1 COMPANY OVERVIEW
17.18.2 GEOGRAPHIC PRESENCE
17.18.3 PRODUCT PORTFOLIO
17.18.4 RECENT DEVELOPMENTS
17.19 SPECTRA LABORATORIES
17.19.1 COMPANY OVERVIEW
17.19.2 GEOGRAPHIC PRESENCE
17.19.3 PRODUCT PORTFOLIO
17.19.4 RECENT DEVELOPMENTS
17.2 DAVITA HEALTHCARE PARTNERS
17.20.1 COMPANY OVERVIEW
17.20.2 GEOGRAPHIC PRESENCE
17.20.3 PRODUCT PORTFOLIO
17.20.4 RECENT DEVELOPMENTS
17.21 ACM MEDICAL LABORATORY
17.21.1 COMPANY OVERVIEW
17.21.2 GEOGRAPHIC PRESENCE
17.21.3 PRODUCT PORTFOLIO
17.21.4 RECENT DEVELOPMENTS
17.22 CEREBA HEALTHCARE
17.22.1 COMPANY OVERVIEW
17.22.2 GEOGRAPHIC PRESENCE
17.22.3 PRODUCT PORTFOLIO
17.22.4 RECENT DEVELOPMENTS
18 RELATED REPORTS
19 CONCLUSION
20 QUESTIONNAIRE
21 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












