Global Clinical Oncology Next Generation Sequencing Market
Market Size in USD Million
CAGR :
% 
 USD
592.56 Million
USD
1,825.30 Million
2021
2029
USD
592.56 Million
USD
1,825.30 Million
2021
2029
| 2022 –2029 | |
| USD 592.56 Million | |
| USD 1,825.30 Million | |
|
|
|
|
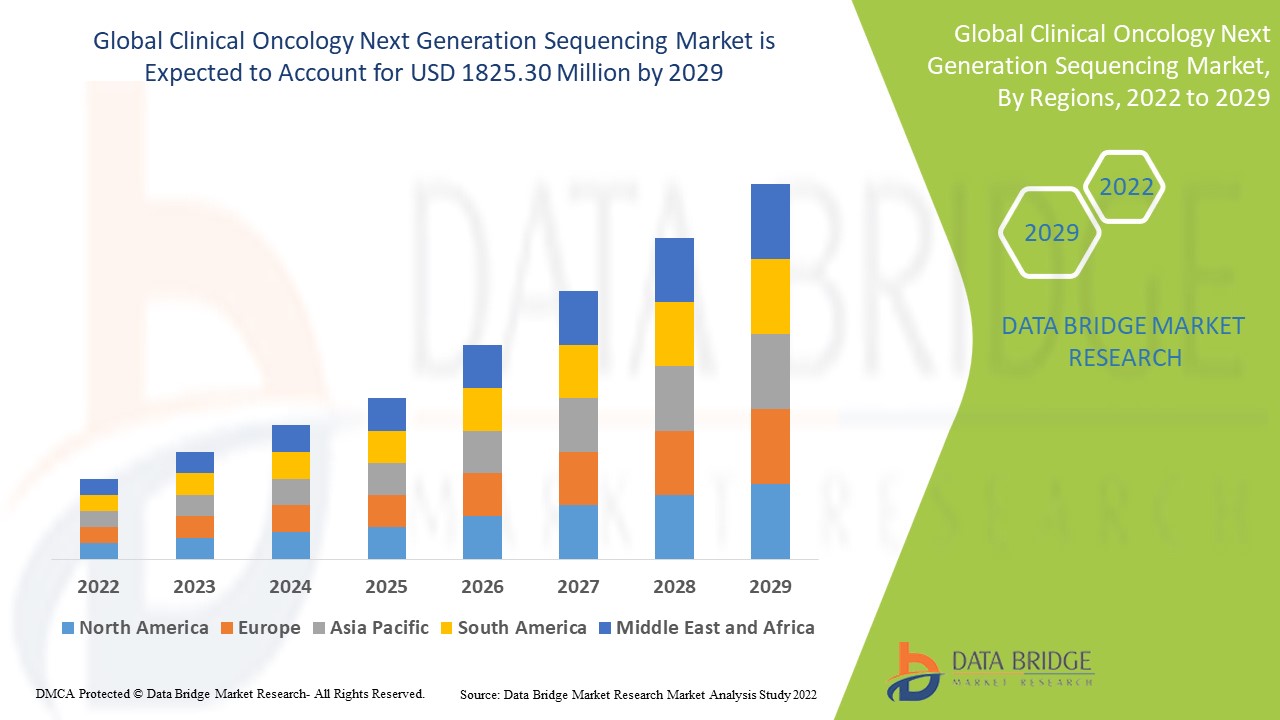
Market Analysis and Size
According to the American Cancer Society, 1.7 million additional cancer cases and 0.6 million cancer deaths occurred in the United States in 2019. Lung, prostate, bladder, and female breast cancer are the four most common cancers worldwide, accounting for 43% of all new cancer cases. As a result, demand for clinical oncology next-generation sequencing is predicted to rise as cancer incidence rates rise globally. Clinical oncology next-generation sequencing companies are increasingly investing in workflow automation to improve accuracy and lower sample-to-sample variability.
Data Bridge Market Research analyses that the clinical oncology next generation sequencing market which was USD 592.56 million in 2021, would rocket up to USD 1825.30 million by 2029, and is expected to undergo a CAGR of 15.1% during the forecast period 2022 to 2029. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Report Scope and Market Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2014 - 2019) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Technology (Whole Genome Sequencing, Whole Exome Sequencing, Targeted Sequencing & Resequencing), Workflow (NGS Pre-Sequencing, NGS Sequencing, NGS Data Analysis), Application (Screening, Companion Diagnostics, Others), End User (Hospitals, Clinics, Laboratories, Others) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Illumina, Inc. (U.S.), Thermo Fisher Scientific Inc. (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), Agilent Technologies Inc. (U.S.), Myriad Genetics Inc. (U.S.), BGI (China), Perkin Elmer Inc. (U.S.), Foundation Medicine Inc. (U.S.), PacBio (U.S.), Oxford Nanopore Technologies plc. (U.K.), Paradigm Diagnostics Inc. (U.S.), Caris Life Sciences (Japan), Partek, Incorporated (U.S.), Eurofins Scientific (Luxembourg), Qiagen (Germany) |
|
Market Opportunities |
|
Market Definition
Next-generation sequencing is a DNA or RNA technique that permits several reactions to be carried out simultaneously. This is one of the most important applications of next-generation sequencing in oncology. In recent years, NGS has changed cancer genetics by providing researchers greater access to genomic and transcriptome data. Next-generation tumour and germline DNA sequencing is used in oncology screening for therapeutic application.
Clinical Oncology Next Generation Sequencing Market Dynamics
Drivers
- Increase in cancer cases
The clinical oncology next-generation sequencing market is projected to be significantly impacted by personalized medicine and oncology improvements. Rising cancer prevalence, high acceptance of sequencing-based diagnostics platforms among practicing oncologists, decrease in the cost of genetic sequencing, and increased government R&D funding are some of the reasons expected to boost market growth. On the other hand, high adoption of this technique over single-gene testing and higher adoption of clinical oncology next-generation sequencing technology and platforms in academia projects will fuel a slew of new opportunities, propelling the clinical oncology next generation sequencing market forward from 2022 to 2029.
- New technological Innovatives
According to the World Health Organization's (WHO) International Agency for Research on Cancer (IARC), cancer was the top cause of death worldwide in 2020, with over 10 million fatalities. In addition, 19.3 million additional cases were reported globally. New creative technologies, such as next generation sequencing, are being used to address this worldwide burden and lower mortality rates. As a result, the clinical oncology next generation market is growing at a faster rate.
- Reduction in the cost of NGS platforms
The factor aiding the market's growth is the reduction in the cost of NGS platforms. Clinical oncology next-generation sequencing approaches provide a high percentage of reads as well as low cost per read. Leading players are introducing low-cost sequencing approaches. Similarly, the worldwide clinical oncology next generation sequencing market will expand over the forecast period as more people use next generation sequencing for liquid biopsy.
Opportunities
Rapidly increasing cancer cases have become a major issue all over the world. The sickness not only kills people but also has a negative influence on countries' economies. As a result, the government and a variety of other healthcare organisations are taking steps to combat cancer's global burden. Research institutes are receiving large sums of money in order to find viable treatments and drugs. New studies are being launched to comprehensively examine the human genome.
Restraints/Challenges
High cost coupled with technology is a major restraint to the growth of the clinical oncology next generation sequencing market in the above mentioned forecast period. High costs connected with the installation of sequencing platforms, poor outsourced service efficiency, and limited availability of sequencing platforms in specific regional markets are all expected to restraint the overall development of the clinical oncology next-generation sequencing market.
This clinical oncology next generation sequencing market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the clinical oncology next generation sequencing market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on Clinical Oncology Next Generation Sequencing Market
The COVID-19 epidemic hugely influenced the world as a whole, causing economic collapse and death. It has resulted in a worldwide pandemic that is extremely transmissible. With the advancement of technology, next-generation sequencing is becoming increasingly important in a variety of fields. From manufacturing PPE kits to inventing test kits to detect the virus and vaccines to prevent transmission, a number of pharmaceutical businesses and agencies have contributed to reducing the infection's impact. To combat the virus, businesses have strengthened their R&D and production workforce due to the increasing benefit of determining and finding the genetic sequence of a virus, which helps scientists to comprehend the virus's entire mutation, the overall influence on the next generation sequencing industry and market is expected to be beneficial.
Recent Development
- In January 2022, Agendia, Inc., a global pioneer in breast cancer precision medicine, announced a multi-year collaboration with Illumina to co-develop in vitro diagnostic (IVD) assays for oncology testing.
- In October 2021, Roche introduced the AVENIO tumour tissue CGP kit to make tailored cancer research more accessible.
Global Clinical Oncology Next Generation Sequencing Market Scope
The clinical oncology next generation sequencing market is segmented on the basis of technology, workflow, application and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Technology
- Whole Genome Sequencing
- Whole Exome Sequencing
- Targeted Sequencing and Resequencing Centrifuges
Workflow
- Pre-Sequencing
- Sequencing
- Data Analysis
Application
- Screening
- Sporadic Cancer
- Inherited Cancer
- Companion Diagnostics
- Other Diagnostics
End-user
- Hospitals
- Clinics
- Laboratories
Clinical Oncology Next Generation Sequencing Market Regional Analysis/Insights
The clinical oncology next generation sequencing market is analysed and market size insights and trends are provided by country, technology, workflow, application and end-user as referenced above.
The countries covered in the clinical oncology next generation sequencing market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the clinical oncology next generation sequencing market due to the initiatives the governments have taken up for these technologies in the countries of this region.
Asia-Pacific is expected to grow at the highest growth rate in the forecast period of 2022 to 2029 due to the increasing automation in this region's pre-sequencing protocols.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Clinical Oncology Next Generation Sequencing Market Share Analysis
The clinical oncology next generation sequencing market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to clinical oncology next generation sequencing market.
Some of the major players operating in the clinical oncology next generation sequencing market are:
- Illumina, Inc. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Agilent Technologies Inc. (U.S.)
- Myriad Genetics Inc. (U.S.)
- BGI (China)
- Perkin Elmer Inc. (U.S.)
- Foundation Medicine Inc. (U.S.)
- PacBio (U.S.)
- Oxford Nanopore Technologies plc. (U.K.)
- Paradigm Diagnostics Inc. (U.S.)
- Caris Life Sciences (Japan)
- Partek, Incorporated (U.S.)
- Eurofins Scientific (Luxembourg)
- Qiagen (Germany)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL CLINICAL ONCOLOGY NEXT GENERATION SEQUENCING MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL CLINICAL ONCOLOGY NEXT GENERATION SEQUENCING MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES VOLUME DATA
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL CLINICAL ONCOLOGY NEXT GENERATION SEQUENCING MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNONLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 FUTURE OUTLOOK
11 REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.2.1 CLASS I
11.2.2 CLASS II
11.2.3 CLASS III
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
12 REIMBURSEMENT FRAMEWORK
13 OPPUTUNITY MAP ANALYSIS
14 VALUE CHAIN ANALYSIS
15 HEALTHCARE ECONOMY
15.1 HEALTHCARE EXPENDITURE
15.2 CAPITAL EXPENDITURE
15.3 CAPEX TRENDS
15.4 CAPEX ALLOCATION
15.5 FUNDING SOURCES
15.6 INDUSTRY BENCHMARKS
15.7 GDP RATION IN OVERALL GDP
15.8 HEALTHCARE SYSTEM STRUCTURE
15.9 GOVERNMENT POLICIES
15.1 ECONOMIC DEVELOPMENT
16 GLOBAL CLINICAL ONCOLOGY NEXT GENERATION SEQUENCING MARKET, BY TECHNOLOGY
16.1 OVERVIEW
16.2 NEXT GENERATION SEQUENCING
16.3 WHOLE GENOME SEQUENCING
16.4 WHOLE EXOME SEQUENCING
16.5 TARGETED SEQUENCING & RESEQUENCING
16.6 OMICS
17 GLOBAL CLINICAL ONCOLOGY NEXT GENERATION SEQUENCING MARKET, BY PRODUCT AND SERVICES
17.1 OVERVIEW
17.2 INSTRUMENTS
17.2.1 BY PRODUCT
17.2.1.1. WORKSTATION/PLATFORM
17.2.1.2. NEXT GENERATION SEQUENCERS
17.2.1.2.1. AUTMATED
17.2.1.2.2. SEMIAUTOMATED
17.2.1.3. OTHERS
17.2.2 BY TYPE
17.2.2.1. HIGH SEQUENCING
17.2.2.2. LOW SEQUENCING
17.2.3 BY MODALITY
17.2.3.1. STANDALONE
17.2.3.2. PORTABLES
17.2.3.3. BENCHTOP
17.3 CONSUMABLES
17.3.1 DNA FRAGMENTATION, END REPAIR, A-TAILING AND SIZE SELECTION
17.3.1.1. REAGENTS AND KITS
17.3.1.1.1. FAST HYBRIDIZATION AND WASH KIT
17.3.1.1.2. STANDARD HYBRIDIZATION AND WASH KIT
17.3.1.1.3. UNIVERSAL BLOCKER
17.3.1.1.4. METHYLATION DETECTION SYSTEM
17.3.1.1.5. OTHERS
17.3.1.2. OTHERS
17.3.2 LIBRARY PREPARATION
17.3.2.1. WHOLE GENOME KITS
17.3.2.1.1. 12 SAMPLE
17.3.2.1.1.1 WITH PREPARATION BEADS
17.3.2.1.1.2 WITHOUT PREPARATION BEADS
17.3.2.1.2. 24 SAMPLE
17.3.2.1.2.1 WITH PREPARATION BEADS
17.3.2.1.2.2 WITHOUT PREPARATION BEADS
17.3.2.1.3. 96 SAMPLE
17.3.2.1.3.1 WITH PREPARATION BEADS
17.3.2.1.3.2 WITHOUT PREPARATION BEADS
17.3.2.2. OTHERS
17.3.3 EXOME GENOMIC KITS
17.3.3.1. 12 SAMPLE
17.3.3.1.1. WITH PREPARATION BEADS
17.3.3.1.2. WITHOUT PREPARATION BEADS
17.3.3.2. 24 SAMPLE
17.3.3.2.1. WITH PREPARATION BEADS
17.3.3.2.2. WITHOUT PREPARATION BEADS
17.3.3.3. 96 SAMPLE
17.3.3.3.1. WITH PREPARATION BEADS
17.3.3.3.2. WITHOUT PREPARATION BEADS
17.3.3.3.3. OTHERS
17.3.4 TARGET ENRICHMENT
17.3.4.1. BY TARGETED REGION
17.3.4.1.1. 60 MB
17.3.4.1.2. 51 MB
17.3.4.1.3. 39 MB
17.3.4.1.4. OTHERS
17.3.4.2. BY PROBE TYPE
17.3.4.2.1. BIOTINYLATED CRNA BAITS
17.3.4.2.2. BIOTINYLATED DNA BAIT
17.3.4.2.3. OTHERS
17.3.4.3. OTHERS
17.4 SERVICES
17.4.1 SEQUENCING SERVICES
17.4.1.1. RNA SEQUENCING
17.4.1.2. WHOLE EXOME SEQUENCING
17.4.1.3. WHOLE GENOME SEQUENCING
17.4.1.4. TARGETED SEQUENCING
17.4.1.5. CHIP SEQUENCING
17.4.1.6. DE NOVO SEQUENCING
17.4.1.7. METHYL SEQUENCING
17.4.1.8. OTHERS
17.4.2 DATA MANAGEMENT SERVICES
17.4.2.1. NGS DATA ANALYSIS SERVICES
17.4.2.2. NGS DATA ANALYSIS SOFTWARE & WORKBENCHES
17.4.2.2.1. LIBRARIES FOR GENOMICS SCREENING
17.4.2.2.2. CRISPR-CAS9 SCREENING LIBRARIES
17.4.2.2.2.1 POOLED SGRNA
17.4.2.2.2.2 ARRAYED CRRNA
17.4.2.2.3. CRISPR ACTIVATION CRRNA LIBRARIES
17.4.2.2.3.1 GENE FAMILIES
17.4.2.2.3.2 DRUGGABLE
17.4.2.2.3.3 WHOLE HUMAN GENOME
17.4.2.2.4. RNAI SCREENING LIBRARIES
17.4.2.2.4.1 SIRNA
17.4.2.2.4.2 SHRNA
17.4.2.2.4.3 MICRORNA
17.4.2.2.5. CDNA AND ORF LIBRARIES
17.4.2.2.5.1 CCSB HUMAN ORFEOME
17.4.2.2.5.2 ORFEOME COLLABORATION
17.4.2.2.5.3 MAMMALIAN GENE COLLECTION
17.4.2.2.6. OTHERS
17.4.2.3. OMICS SERVICES
17.4.2.4. OTHERS
18 GLOBAL CLINICAL ONCOLOGY NEXT GENERATION SEQUENCING MARKET, BY SEQUENCING TYPE
18.1 OVERVIEW
18.2 SINGLE-READ SEQUENCING
18.3 PAIRED-END SEQUENCING
19 GLOBAL CLINICAL ONCOLOGY NEXT GENERATION SEQUENCING MARKET, BY METHODS OF SEQUENCING
19.1 OVERVIEW
19.2 LOW THROUGHPUT
19.3 MEDIUM THROUGHPUT
19.4 HIGH THROUGHPUT
20 GLOBAL CLINICAL ONCOLOGY NEXT GENERATION SEQUENCING MARKET, BY APPLICATION
20.1 OVERVIEW
20.2 PRECISION MEDICINE FOR CANCER
20.3 LIQUID BIOPSY
20.4 MINIMAL RESIDUAL DISEASE (MRD) MONITORING
20.5 CANCER IMMUNOTHERAPY
20.6 OTHERS
21 GLOBAL CLINICAL ONCOLOGY NEXT GENERATION SEQUENCING MARKET, BY CANCER TYPE
21.1 OVERVIEW
21.2 BREAST CANCER
21.3 LUNG CANCER
21.4 COLORECTAL CANCER
21.5 PROSTATE CANCER
21.6 LEUKEMIA
21.7 LYMPHOMA
21.8 MELANOMA
21.9 OTHERS
22 GLOBAL CLINICAL ONCOLOGY NEXT GENERATION SEQUENCING MARKET, BY END USER
22.1 OVERVIEW
22.2 HOSPITALS
22.2.1 PUBLIC
22.2.2 PRIVATE
22.3 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
22.3.1 SMALL
22.3.2 MEDIUM
22.3.3 LARGE
22.4 ACADEMIC & RESEARCH INSTITUTES
22.5 CLINICAL LABORATORIES
22.6 GOVERNMENT INSTITUTES
22.7 FORENSIC LABORATORIES
22.8 CONTRACT RESEARCH ORGANIZATIONS (CROS)
22.9 OTHERS
23 GLOBAL CLINICAL ONCOLOGY NEXT GENERATION SEQUENCING MARKET, BY DISTRUBUTION CHANNEL
23.1 OVERVIEW
23.2 DIRECT TENDERS
23.3 RETAIL SALES
23.4 OTHERS
24 GLOBAL CLINICAL ONCOLOGY NEXT GENERATION SEQUENCING MARKET, BY REGION
24.1 GLOBAL MEDICAL CARTS MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
24.2 NORTH AMERICA
24.2.1 U.S.
24.2.2 CANADA
24.2.3 MEXICO
24.3 EUROPE
24.3.1 GERMANY
24.3.2 FRANCE
24.3.3 U.K.
24.3.4 ITALY
24.3.5 SPAIN
24.3.6 RUSSIA
24.3.7 TURKEY
24.3.8 BELGIUM
24.3.9 NETHERLANDS
24.3.10 SWITZERLAND
24.3.11 REST OF EUROPE
24.4 ASIA-PACIFIC
24.4.1 JAPAN
24.4.2 CHINA
24.4.3 SOUTH KOREA
24.4.4 INDIA
24.4.5 AUSTRALIA
24.4.6 SINGAPORE
24.4.7 THAILAND
24.4.8 MALAYSIA
24.4.9 INDONESIA
24.4.10 PHILIPPINES
24.4.11 REST OF ASIA-PACIFIC
24.5 SOUTH AMERICA
24.5.1 BRAZIL
24.5.2 ARGENTINA
24.5.3 REST OF SOUTH AMERICA
24.6 MIDDLE EAST AND AFRICA
24.6.1 SOUTH AFRICA
24.6.2 SAUDI ARABIA
24.6.3 UAE
24.6.4 EGYPT
24.6.5 ISRAEL
24.6.6 REST OF MIDDLE EAST AND AFRICA
24.7 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
25 GLOBAL CLINICAL ONCOLOGY NEXT GENERATION SEQUENCING MARKET, COMPANY LANDSCAPE
25.1 COMPANY SHARE ANALYSIS: GLOBAL
25.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
25.3 COMPANY SHARE ANALYSIS: EUROPE
25.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
25.5 MERGERS & ACQUISITIONS
25.6 NEW PRODUCT DEVELOPMENT & APPROVALS
25.7 EXPANSIONS & PARTNERSHIP
25.8 REGULATORY CHANGES
26 GLOBAL CLINICAL ONCOLOGY NEXT GENERATION SEQUENCING MARKET, SWOT AND DBMR ANALYSIS
27 GLOBAL CLINICAL ONCOLOGY NEXT GENERATION SEQUENCING MARKET, COMPANY PROFILE
27.1 ACT GENOMICS
27.1.1 COMPANY OVERVIEW
27.1.2 REVENUE ANALYSIS
27.1.3 GEOGRAPHIC PRESENCE
27.1.4 PRODUCT PORTFOLIO
27.1.5 RECENT DEVELOPMENTS
27.2 RESOLUTION BIOSCIENCE, INC. (AGILENT TECHNOLOGIES, INC.)
27.2.1 COMPANY OVERVIEW
27.2.2 REVENUE ANALYSIS
27.2.3 GEOGRAPHIC PRESENCE
27.2.4 PRODUCT PORTFOLIO
27.2.5 RECENT DEVELOPMENTS
27.3 ILLUMINA, INC.
27.3.1 COMPANY OVERVIEW
27.3.2 REVENUE ANALYSIS
27.3.3 GEOGRAPHIC PRESENCE
27.3.4 PRODUCT PORTFOLIO
27.3.5 RECENT DEVELOPMENTS
27.4 BD
27.4.1 COMPANY OVERVIEW
27.4.2 REVENUE ANALYSIS
27.4.3 GEOGRAPHIC PRESENCE
27.4.4 PRODUCT PORTFOLIO
27.4.5 RECENT DEVELOPMENTS
27.5 BERRY GENOMICS
27.5.1 COMPANY OVERVIEW
27.5.2 REVENUE ANALYSIS
27.5.3 GEOGRAPHIC PRESENCE
27.5.4 PRODUCT PORTFOLIO
27.5.5 RECENT DEVELOPMENTS
27.6 BIO-RAD LABORATORIES, INC.
27.6.1 COMPANY OVERVIEW
27.6.2 REVENUE ANALYSIS
27.6.3 GEOGRAPHIC PRESENCE
27.6.4 PRODUCT PORTFOLIO
27.6.5 RECENT DEVELOPMENTS
27.7 DNASTAR
27.7.1 COMPANY OVERVIEW
27.7.2 REVENUE ANALYSIS
27.7.3 GEOGRAPHIC PRESENCE
27.7.4 PRODUCT PORTFOLIO
27.7.5 RECENT DEVELOPMENTS
27.8 EUROFINS GENOMICS
27.8.1 COMPANY OVERVIEW
27.8.2 REVENUE ANALYSIS
27.8.3 GEOGRAPHIC PRESENCE
27.8.4 PRODUCT PORTFOLIO
27.8.5 RECENT DEVELOPMENTS
27.9 QIAGEN
27.9.1 COMPANY OVERVIEW
27.9.2 REVENUE ANALYSIS
27.9.3 GEOGRAPHIC PRESENCE
27.9.4 PRODUCT PORTFOLIO
27.9.5 RECENT DEVELOPMENTS
27.1 CARIS LIFE SCIENCES
27.10.1 COMPANY OVERVIEW
27.10.2 REVENUE ANALYSIS
27.10.3 GEOGRAPHIC PRESENCE
27.10.4 PRODUCT PORTFOLIO
27.10.5 RECENT DEVELOPMENTS
27.11 F. HOFFMANN-LA ROCHE LTD
27.11.1 COMPANY OVERVIEW
27.11.2 REVENUE ANALYSIS
27.11.3 GEOGRAPHIC PRESENCE
27.11.4 PRODUCT PORTFOLIO
27.11.5 RECENT DEVELOPMENTS
27.12 THERMO FISHER SCIENTIFIC INC
27.12.1 COMPANY OVERVIEW
27.12.2 REVENUE ANALYSIS
27.12.3 GEOGRAPHIC PRESENCE
27.12.4 PRODUCT PORTFOLIO
27.12.5 RECENT DEVELOPMENTS
27.13 MYRIAD GENETICS, INC
27.13.1 COMPANY OVERVIEW
27.13.2 REVENUE ANALYSIS
27.13.3 GEOGRAPHIC PRESENCE
27.13.4 PRODUCT PORTFOLIO
27.13.5 RECENT DEVELOPMENTS
27.14 BGI
27.14.1 COMPANY OVERVIEW
27.14.2 REVENUE ANALYSIS
27.14.3 GEOGRAPHIC PRESENCE
27.14.4 PRODUCT PORTFOLIO
27.14.5 RECENT DEVELOPMENTS
27.15 REVVITY
27.15.1 COMPANY OVERVIEW
27.15.2 REVENUE ANALYSIS
27.15.3 GEOGRAPHIC PRESENCE
27.15.4 PRODUCT PORTFOLIO
27.15.5 RECENT DEVELOPMENTS
27.16 FOUNDATION MEDICINE, INC.,
27.16.1 COMPANY OVERVIEW
27.16.2 REVENUE ANALYSIS
27.16.3 GEOGRAPHIC PRESENCE
27.16.4 PRODUCT PORTFOLIO
27.16.5 RECENT DEVELOPMENTS
27.17 PACBIO
27.17.1 COMPANY OVERVIEW
27.17.2 REVENUE ANALYSIS
27.17.3 GEOGRAPHIC PRESENCE
27.17.4 PRODUCT PORTFOLIO
27.17.5 RECENT DEVELOPMENTS
27.18 OXFORD NANOPORE TECHNOLOGIES PLC.
27.18.1 COMPANY OVERVIEW
27.18.2 REVENUE ANALYSIS
27.18.3 GEOGRAPHIC PRESENCE
27.18.4 PRODUCT PORTFOLIO
27.18.5 RECENT DEVELOPMENTS
27.19 GRAPHPAD SOFTWARE, LLC,
27.19.1 COMPANY OVERVIEW
27.19.2 REVENUE ANALYSIS
27.19.3 GEOGRAPHIC PRESENCE
27.19.4 PRODUCT PORTFOLIO
27.19.5 RECENT DEVELOPMENTS
27.2 MACROGEN, INC.
27.20.1 COMPANY OVERVIEW
27.20.2 REVENUE ANALYSIS
27.20.3 GEOGRAPHIC PRESENCE
27.20.4 PRODUCT PORTFOLIO
27.20.5 RECENT DEVELOPMENTS
27.21 AZENTA LIFE SCIENCES
27.21.1 COMPANY OVERVIEW
27.21.2 REVENUE ANALYSIS
27.21.3 GEOGRAPHIC PRESENCE
27.21.4 PRODUCT PORTFOLIO
27.21.5 RECENT DEVELOPMENTS
27.22 TAKARA BIO INC.
27.22.1 COMPANY OVERVIEW
27.22.2 REVENUE ANALYSIS
27.22.3 GEOGRAPHIC PRESENCE
27.22.4 PRODUCT PORTFOLIO
27.22.5 RECENT DEVELOPMENTS
27.23 NOVOGENE CO, LTD.
27.23.1 COMPANY OVERVIEW
27.23.2 REVENUE ANALYSIS
27.23.3 GEOGRAPHIC PRESENCE
27.23.4 PRODUCT PORTFOLIO
27.23.5 RECENT DEVELOPMENTS
27.24 STANDARD BIOTOOLS INC.
27.24.1 COMPANY OVERVIEW
27.24.2 REVENUE ANALYSIS
27.24.3 GEOGRAPHIC PRESENCE
27.24.4 PRODUCT PORTFOLIO
27.24.5 RECENT DEVELOPMENTS
27.25 CREATIVE BIOLABS
27.25.1 COMPANY OVERVIEW
27.25.2 REVENUE ANALYSIS
27.25.3 GEOGRAPHIC PRESENCE
27.25.4 PRODUCT PORTFOLIO
27.25.5 RECENT DEVELOPMENTS
27.26 ZYMO RESEARCH CORPORATION
27.26.1 COMPANY OVERVIEW
27.26.2 REVENUE ANALYSIS
27.26.3 GEOGRAPHIC PRESENCE
27.26.4 PRODUCT PORTFOLIO
27.26.5 RECENT DEVELOPMENTS
27.27 VERACYTE, INC
27.27.1 COMPANY OVERVIEW
27.27.2 REVENUE ANALYSIS
27.27.3 GEOGRAPHIC PRESENCE
27.27.4 PRODUCT PORTFOLIO
27.27.5 RECENT DEVELOPMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
28 RELATED REPORTS
29 CONCLUSION
30 QUESTIONNAIRE
31 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.













