Global Clinical Trial Management System Market
Market Size in USD Billion
CAGR :
% 
 USD
1.41 Billion
USD
3.87 Billion
2024
2032
USD
1.41 Billion
USD
3.87 Billion
2024
2032
| 2025 –2032 | |
| USD 1.41 Billion | |
| USD 3.87 Billion | |
|
|
|
|
Clinical Trial Management System (CTMS) Market Size
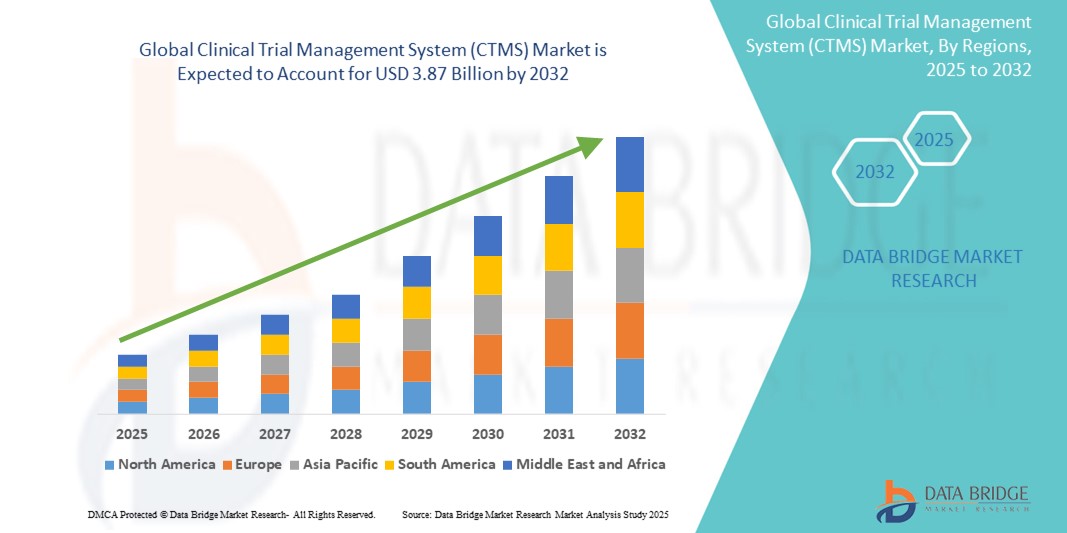
- The global clinical trial management system (CTMS) market size was valued at USD 1.41 billion in 2024 and is expected to reach USD 3.87 billion by 2032, at a CAGR of 13.4% during the forecast period
- This growth is driven by factors such as increasing R&D investments, growing clinical trials volume and technological advancements
Clinical Trial Management System (CTMS) Market Analysis
- Clinical trial management systems (CTMS) are critical tools in the pharmaceutical and biotechnology industries, providing comprehensive solutions for planning, tracking, and managing clinical trials. They play a vital role in ensuring regulatory compliance, improving data accuracy, and streamlining trial operations
- The demand for CTMS solutions is significantly driven by the increasing volume of clinical trials, rising R&D investments, and the growing complexity of clinical studies. The integration of advanced technologies, including AI, machine learning, and cloud computing, is further enhancing the efficiency and scalability of CTMS platforms
- North America is expected to dominate the clinical trial management system (CTMS) market, accounting for approximately 47.6% of the global market share, driven by the presence of major pharmaceutical and biotechnology companies, strong clinical research infrastructure, and favorable government regulations supporting clinical trials
- Asia-Pacific is expected to be the fastest growing region in the clinical trial management system (CTMS) market, with a CAGR of 12.8%, driven by increasing clinical trial activity, rapid expansion of healthcare infrastructure, and cost advantages for conducting clinical research in the region
- Web-based segment is expected to dominate the market with the largest share of 71.7%, driven by its scalability, flexibility, and cost-effectiveness. As the preferred choice for managing clinical trials, cloud-based CTMS platforms provide real-time access to trial data, enhanced data security, and reduced IT infrastructure costs, supporting efficient trial management
Report Scope and Clinical Trial Management System (CTMS) Market Segmentation
|
Attributes |
Clinical Trial Management System (CTMS) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Clinical Trial Management System (CTMS) Market Trends
“Integration of AI and Advanced Analytics in CTMS”
- One significant trend in the evolution of clinical trial management systems is the growing integration of artificial intelligence (AI), machine learning (ML), and advanced analytics
- These technologies enable real-time data analysis, predictive insights, and process automation, enhancing the efficiency and accuracy of clinical trial management
- For instance, AI-powered CTMS platforms can predict patient enrollment challenges, optimize site selection, and identify potential delays, significantly reducing trial timelines and costs. This capability is particularly valuable in large-scale, multinational trials where rapid decision-making is critical
- These advancements are transforming clinical trial operations, improving patient outcomes, and driving the demand for next-generation CTMS platforms with cutting-edge data analytics and AI integration
Clinical Trial Management System (CTMS) Market Dynamics
Driver
“Growing Demand for Efficient Clinical Trial Management”
- The increasing complexity of clinical trials, driven by the rise of precision medicine, personalized therapies, and decentralized trials, is significantly contributing to the demand for robust clinical trial management systems
- As clinical trials become more global and data-intensive, the need for real-time data access, streamlined workflows, and regulatory compliance becomes critical for sponsors, CROs, and research institutions
- Advanced CTMS platforms offer integrated solutions for site management, patient enrollment, data collection, and financial tracking, enhancing trial efficiency and reducing operational costs
For instance,
- In January 2024, according to an article published in the Journal of Clinical Research, the global clinical trials market is projected to grow significantly, with over 30% of trials expected to adopt decentralized models by 2028. This shift is driving the demand for advanced CTMS solutions that can handle complex trial designs and remote data management
- As a result, the need for efficient clinical trial management systems is expected to increase, supporting the growth of the CTMS market
Opportunity
“Integration of AI and Advanced Analytics in CTMS”
- AI-powered CTMS platforms can enhance trial efficiency by automating routine tasks, improving patient recruitment, and optimizing data analysis, enabling more accurate and faster decision-making
- These platforms leverage machine learning algorithms to predict trial delays, optimize site selection, and reduce dropout rates, significantly reducing trial timelines and costs
- In addition, AI-driven CTMS systems can provide real-time insights into patient data, enabling more personalized and adaptive trial designs
For instance,
- In March 2025, according to a report by Clinical Research News, several leading pharma companies have integrated AI into their CTMS platforms, resulting in up to 40% faster patient recruitment and a 30% reduction in trial costs. These advancements are expected to transform clinical trial management, driving demand for AI-enabled CTMS solutions
- The integration of AI in CTMS systems can also improve data accuracy, reduce operational costs, and enhance overall trial outcomes, creating significant growth opportunities for the market
Restraint/Challenge
“High Implementation Costs and Data Security Concerns”
- The high cost of implementing and maintaining advanced CTMS platforms can pose a significant barrier for small and mid-sized clinical research organizations (CROs) and biotech firms
- These platforms often require substantial upfront investment, including software licensing, infrastructure upgrades, and staff training, which can be prohibitive for smaller organizations
- In addition, concerns over data security and regulatory compliance can further hinder adoption, as clinical trials generate sensitive patient data that must be protected from breaches and unauthorized access
For instance,
- In September 2024, a report by Data Protection Journal highlighted the increasing threat of cyberattacks in the healthcare sector, with clinical trial data becoming a prime target. This has prompted many organizations to invest heavily in cybersecurity, adding to the overall cost of CTMS implementation
- As a result, the high cost and data security challenges associated with CTMS systems can limit their adoption, particularly among smaller organizations, slowing overall market growth
Clinical Trial Management System (CTMS) Market Scope
The market is segmented on the basis of type, delivery, components and end user.
|
Segmentation |
Sub-Segmentation |
|
By Type |
|
|
By Delivery |
|
|
By Components |
|
|
By End User |
|
In 2025, the web-based is projected to dominate the market with a largest share in delivery segment
The web-based delivery segment is expected to dominate the clinical trial management system (CTMS) market with the largest share of 71.7% in 2025 due to its scalability, flexibility, and cost-effectiveness. As the preferred choice for managing clinical trials, cloud-based CTMS platforms provide real-time access to trial data, enhanced data security, and reduced IT infrastructure costs, supporting efficient trial management. The ongoing shift toward decentralized trials and remote patient monitoring further contributes to the dominance of this segment, driving market growth.
The enterprise is expected to account for the largest share during the forecast period in type market
In 2025, the enterprise segment is expected to dominate the clinical trial management system (CTMS) market with the largest market share of 65.4% due to its ability to manage complex, multi-site clinical trials and support global operations. As the leading choice for large-scale clinical research, enterprise CTMS platforms offer real-time data access, robust analytics, and seamless collaboration among clinical trial stakeholders, enhancing trial efficiency and outcomes. The growing demand for integrated, scalable solutions further contributes to its market dominance.
Clinical Trial Management System (CTMS) Market Regional Analysis
“North America Holds the Largest Share in the Clinical Trial Management System (CTMS) Market”
- North America is expected to dominate the clinical trial management system (CTMS) market, accounting for 47.6% of the global market share, driven by the presence of major pharmaceutical and biotechnology companies, strong clinical research infrastructure, and favorable government regulations supporting clinical trials
- U.S. holds a significant share of 35.3% due to the high volume of clinical trials, substantial R&D investments, and the early adoption of advanced clinical trial management technologies
- The region's well-established healthcare ecosystem, extensive patient population, and focus on personalized medicine further contribute to its market leadership.
- In addition, the presence of skilled professionals, advanced IT infrastructure, and strong regulatory frameworks further strengthen the CTMS market in North America
“Asia-Pacific is Projected to Register the Highest CAGR in the Clinical Trial Management System (CTMS) Market”
- Asia-Pacific is expected to witness the highest growth rate in the Clinical Trial Management System (CTMS) market, with a projected CAGR of approximately 12.8%, driven by increasing clinical trial activity, rapid expansion of healthcare infrastructure, and cost advantages for conducting clinical research in the region
- Countries such as China, India, and South Korea are emerging as key markets due to the growing pharmaceutical and biotechnology industries and increasing patient enrollment in clinical trials
- China leads the regional market with significant government support for clinical research, large patient populations, and expanding biopharmaceutical manufacturing capabilities
- India is projected to register the highest CAGR in the CTMS market, driven by cost-efficient clinical trial operations, a growing number of contract research organizations (CROs), and increasing foreign investments in the healthcare sector
Clinical Trial Management System (CTMS) Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Advarra (U.S.)
- ICON plc (Ireland)
- Merative (U.S.)
- DSG, Inc. (U.S.)
- ArisGlobal (U.S.)
- Clario (U.S.)
- Oracle (U.S.)
- Medidata (U.S.)
- Fountayn (U.S.)
- MedNet (U.S.)
- IQVIA Inc. (U.S.)
- SimpleTrials (U.S.)
- Calyx Global Inc. (U.S.)
- RealTime Software Solutions, LLC (U.S.)
- LabCorp (U.S.)
- Veeva Systems (U.S.)
- Wipro (India)
- PHARMASEAL (U.K.)
Latest Developments in Global Clinical Trial Management System (CTMS) Market
- In February 2025, Medidata Solutions, a leading provider of clinical trial technology, announced the launch of its new AI-powered Clinical Trial Management System designed to enhance data integration and real-time analytics. The system offers improved trial monitoring capabilities, automated risk assessments, and streamlined regulatory compliance workflows, aimed at accelerating drug development timelines and improving trial efficiency
- In November 2024, Oracle Corporation unveiled the latest version of its CTMS platform with enhanced cloud-based features, including advanced patient recruitment tools, remote monitoring support, and integrated eConsent modules. These innovations are intended to support decentralized and hybrid clinical trials, improving participant engagement and data accuracy
- In October 2024, IQVIA Inc. introduced its next-generation CTMS solution incorporating machine learning algorithms to predict trial risks and optimize resource allocation. The platform also provides real-time dashboards and customizable reports to improve decision-making for clinical research organizations (CROs) and sponsors
- In August 2024, Clario announced an expansion of its CTMS offerings with integrated electronic data capture (EDC) and patient-reported outcomes (PRO) modules. This unified system aims to simplify data management, enhance compliance, and improve patient-centric trial designs. The updated platform targets improved operational efficiency and regulatory readiness
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.