Global Fibrodysplasia Ossificans Progressiva Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
500.00 Million
USD
684.28 Million
2022
2030
USD
500.00 Million
USD
684.28 Million
2022
2030
| 2023 –2030 | |
| USD 500.00 Million | |
| USD 684.28 Million | |
|
|
|
|
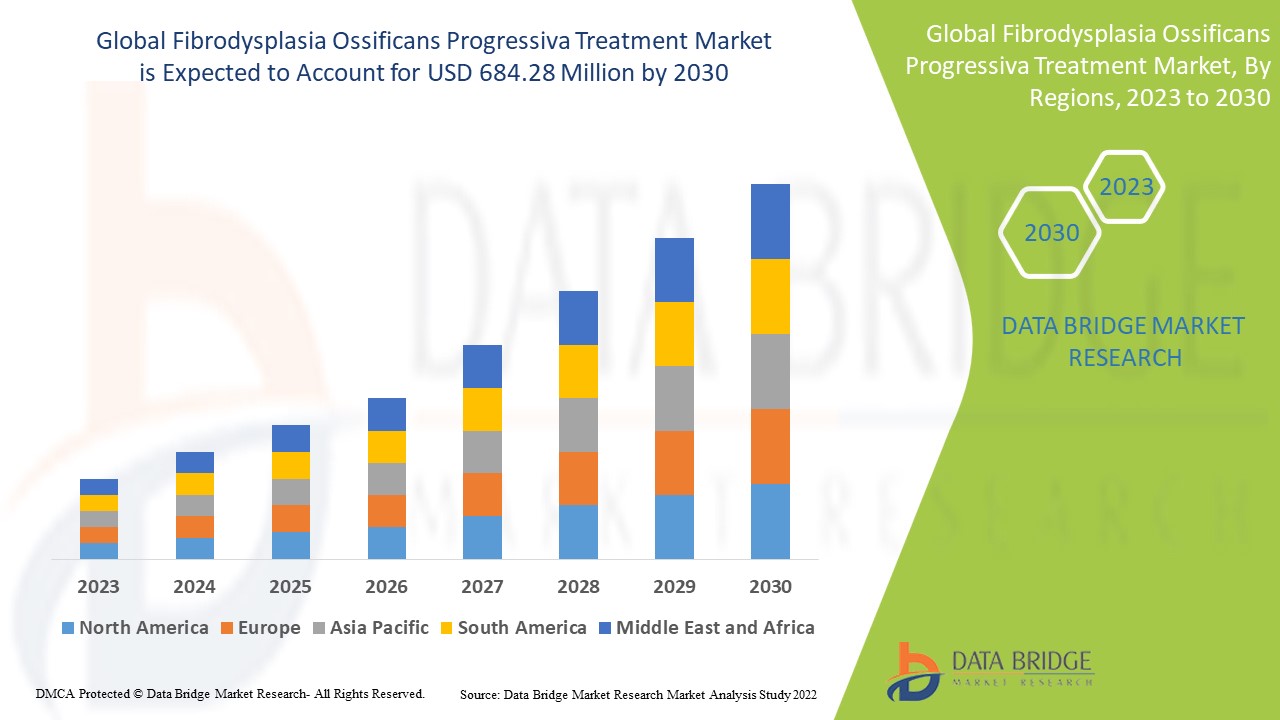
Fibrodysplasia Ossificans Progressiva Treatment Market Analysis and Size
Fibrodysplasia ossificans progressiva (FOP) is a rare hereditary disorder. The market is expected to expand rapidly in the few years. Introduction of novel therapies and rise in cases of fibrodysplasia ossificans progressiva are likely to be major drivers of the market. Increase in demand for the treatment of this disease and increase in awareness associated with FOP are also projected to drive the market during the forecast period.
Data Bridge Market Research analyses a growth rate in the fibrodysplasia ossificans progressiva treatment market in the forecast period 2023-2030. The expected CAGR of fibrodysplasia ossificans progressiva treatment market tend to be around 4% in the mentioned forecast period. The market was valued at USD 500 million in 2022 and it would grow upto USD 684.28 million by 2030. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Fibrodysplasia Ossificans Progressiva Treatment Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Drugs Class (Corticosteroids, NSAIDs, Bisphosphonates and Others), Drugs (Rituximab, Ascorbic Acid Acetic Acid, and Others), Treatment (Medication, Occupational Therapy, and Surgery), Route of Administration (Oral, Topical and Parenteral),End-User (Hospitals, Research Institutes, Specialty Clinics), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, online Pharmacies and Others) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Pfizer Inc. (U.S.), Abbvie Inc. (U.S.), Novartis AG (Switzerland), Amgen Inc. (U.S.), Boehringer Ingelheim International GmbH. (Germany), Merck & Co Inc. (U.S.), Sun Pharmaceutical Industries Ltd. (India), Teva Pharmaceuticals Industries Ltd. (Israel), AstraZeneca (U.K.), Lilly (U.S.), Zydus Group (India), Fresenius Kabi AG (Germany), Accord Healthcare, Inc. (U.S.), DAIICHI SANKYO COMPANY, LIMITED (Japan), Regeneron Pharmaceuticals Inc (U.S.), Blueprint Medicines Corporation (U.S.), Ipsen Pharma (France), BIOCRYST PHARMACEUTICALS, INC. (U.S.), NATCO Pharma Limited (India), Thermo Fisher Scientific (U.S.), bioMérieux S.A. (France), Bayer AG (Germany), Bristol Myers Squibb Company (U.S.), and Abbott (U.S.) |
|
Market Opportunities |
|
Market Definition
Fibrodysplasia ossificans progressiva (FOP) is a type of rare genetic disorder wherein the muscle tissue and connective tissue, such as the tendons and ligaments are substituted by bone, forming bone outside the skeleton that limits the movement of that specific part of the body. This can cause malformations such as shortened great toe with a malformed distal first metatarsal, abnormally short fingers and toes, inward turning of the great toe toward the other toes, or permanent fixation of the fifth finger in a bent position.
Fibrodysplasia Ossificans Progressiva Treatment Market Dynamics
Drivers
- Growing Incidence of Fibrodysplasia ossificans progressiva treatment
As per the Journal of Rare Disease Research and Treatment, in 2016, the incidence of FOP ranged considerably from around 0.65 per million in North America, 0.47 per million in Western Europe, and 0.27 per million in Latin America to 0.05 per million in Africa and nearly 0.04 per million in the Asia Pacific. Therefore, it acts as a major driver in the market growth.
- Increase in the number of R&D activities
The market's growth is fuelled by the increase in the number of R&D activities. This will provide much beneficial opportunities for the market. For instance, in January 2020, Regeneron Pharmaceuticals, Inc., which is an American pharmaceutical company, showcased the results from a Phase 2, double-blind placebo-controlled trial, LUMINA-1, the trial was originated to assess garetosmab for the treatment of fibrodysplasia ossificans progressiva.
Opportunities
- Increased Partnerships and Collaborations
There have been increased partnerships and collaborations between market players that lead to the growth of the market. For instance, In October 2019, Clementia Pharmaceuticals, a subsidiary of Ipsen, and Blueprint Medicines Corporation came into an exclusive license agreement to commercialize and develop BLU-782, which is a highly selective investigational ALK2 inhibitor, that is indicated for the treatment of fibrodysplasia ossificans progressiva (FOP).
Restraints/Challenges
- Lack of Awareness
The sales of potential products are not succeeding much due to the lack of awareness of the syndrome. Though effective treatments are available, but poor inadequate communication between clinicians and patients, treatment adherence impedes market growth.
- High Cost
The enormous expenditure associated with the treatment processes hinders market growth. Various market players make huge investments in installing new and advanced treatment procedures to faster the recovery process and in return the cost is increased.
This fibrodysplasia ossificans progressiva treatment market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the fibrodysplasia ossificans progressiva treatment market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Global Fibrodysplasia Ossificans Progressiva Treatment Market Scope
The fibrodysplasia ossificans progressiva treatment market is segmented on the basis of drug class, drug, treatment, route of administration, distribution channel and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Drug Class
- Corticosteroids
- NSAIDs
- Bisphosphonates
- Others
Drugs
- Rituximab
- Ascorbic Acid
- Acetic Acid
- Others
Treatment
- Medication
- Occupational Therapy
- Surgery
Route of Administration
- Oral
- Topical
- Parenteral
End-User
- Hospitals
- Research Institutes
- Specialty Clinics
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacies
- Others
Fibrodysplasia Ossificans Progressiva Treatment Market Regional Analysis/Insights
The fibrodysplasia ossificans progressiva treatment market is analyzed and market size insights and trends are provided by drug class, drug treatment, distribution channel, route of administration, and end-user as referenced above.
The major countries covered in the fibrodysplasia ossificans progressiva treatment market report are the U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the market because of high per capita income, favorable healthcare infrastructure, and high rate of adoption of advanced surgeries in the region.
Asia-Pacific is considered to have the most lucrative period due to the rise in cases of connective tissue disorders.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Global Fibrodysplasia Ossificans Progressiva Treatment Market Share Analysis
The fibrodysplasia ossificans progressiva treatment market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to fibrodysplasia ossificans progressiva treatment market
Key players operating in the fibrodysplasia ossificans progressiva treatment market include:
- Pfizer Inc. (U.S.)
- Abbvie Inc. (U.S.)
- Novartis AG (Switzerland)
- Amgen Inc. (U.S.)
- Boehringer Ingelheim International GmbH. (Germany)
- Merck & Co Inc. (U.S.)
- Sun Pharmaceutical Industries Ltd. (India)
- Teva Pharmaceuticals Industries Ltd. (Israel)
- AstraZeneca (U.K.)
- Lilly (U.S.)
- Zydus Group (India)
- Fresenius Kabi AG (Germany)
- Accord Healthcare, Inc. (U.S.)
- DAIICHI SANKYO COMPANY, LIMITED (Japan)
- Regeneron Pharmaceuticals Inc (U.S.)
- Blueprint Medicines Corporation (U.S.)
- Ipsen Pharma (France)
- BIOCRYST PHARMACEUTICALS, INC. (U.S.)
- NATCO Pharma Limited (India)
- Thermo Fisher Scientific (U.S.)
- bioMérieux S.A. (France)
- Bayer AG (Germany)
- Bristol Myers Squibb Company (U.S.)
- Abbott (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL FIBRODYSPLASIA OSSIFICANS PROGRESSIVA TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL FIBRODYSPLASIA OSSIFICANS PROGRESSIVA TREATMENT MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL FIBRODYSPLASIA OSSIFICANS PROGRESSIVA TREATMENT MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S 5 FORCES
5.3 GLOBAL FIBRODYSPLASIA OSSIFICANS PROGRESSIVA TREATMENT MARKET ANALYSIS
6 EPIDEMOLOGY
6.1 EPIDEMOLOGY OF FIBRODYSPLASIA OSSIFICANS PROGRESSIVA
6.2 SUMMARY OF THE FEATURES OF THE REPORTED PATIENTS
6.3 COUNTRY WISE NO OF PATIENTS AS PER THE FIBRODYSPLASIA OSSIFICANS PROGRESSIVA COMMUNITY
6.4 LIST OF COUNTRIES WITH HIGHEST PREVELANCE OF FIBRODYSPLASIA OSSIFICANS PROGRESSIVA
7 PIPELINE ANALYSIS
7.1 PHASE 1
7.2 PHASE 2
7.3 PHASE 3
7.4 PRECLINICAL
8 INDUSTRY INSIGHTS
8.1 DEMOGRAPHIC TRENDS
8.2 KEY PRICING STRATEGIES
8.3 KEY PATIENT ENROLLMENT STRATEGIES
8.4 INTERVIEWS WITH MANUFACTURING COMPANIES
8.5 OTHER KOL SNAPSHOTS
9 REGULATORY FRAMWORK
10 GLOBAL FIBRODYSPLASIA OSSIFICANS PROGRESSIVA TREATMENT MARKET, BY TREATMENT
10.1 OVERVIEW
(NOTE: MARKET VALUE, MARKET VOLUME AND ASP WILL BE PROVIDED FOR ALL SEGMENTS AND SUB SEGMENTS)
10.2 MEDICATION
10.2.1 OFF LABEL DRUGS
10.2.1.1. CORTICOSTEROIDS
10.2.1.2. NSAIDS
10.2.1.2.1. CELECOXIB
10.2.1.2.2. IBUPROFEN
10.2.1.2.3. INDOMETHACIN
10.2.1.2.4. OTHERS
10.2.1.3. BISPHOSPHONATES
10.2.1.3.1. ZOLEDRONATE
10.2.1.3.2. PAMIDRONATE
10.2.1.3.3. OTHERS
10.2.1.4. MUSCLE RELAXANT
10.2.1.4.1. CYCLOBENZAPRINE
10.2.1.4.2. METAXALONE
10.2.1.4.3. LIORESAL
10.2.1.4.4. OTHERS
10.2.1.5. OTHERS
10.2.2 PIPELLINE DRUGS
10.2.2.1. PALOVAROTENE
10.2.2.2. GARETOSMAB
10.2.2.3. INCB000928
10.2.2.4. IPN60130
10.2.2.5. RAPAMYCIN
10.2.2.6. SARACATINIB
10.2.2.7. BCX9250
10.2.2.8. DS-6016A
10.2.2.9. KER-047
10.2.2.10. OTHERS
10.3 SURGERY
11 GLOBAL FIBRODYSPLASIA OSSIFICANS PROGRESSIVA TREATMENT MARKET, BY ROUTE OF ADMINISTRATION
11.1 OVERVIEW
11.2 ORAL
11.2.1 TABLET
11.2.2 CAPSULE
11.2.3 OTHERS
11.3 TOPICAL
11.4 PARENTRAL
12 GLOBAL FIBRODYSPLASIA OSSIFICANS PROGRESSIVA TREATMENT MARKET, BY AGE
12.1 OVERVIEW
12.2 0-30 YRS
12.3 31-50
12.4 ABOVE 50 YRS
13 GLOBAL FIBRODYSPLASIA OSSIFICANS PROGRESSIVA TREATMENT MARKET, BY GENDER
13.1 OVERVIEW
13.2 MALE
13.3 FEMALE
14 GLOBAL FIBRODYSPLASIA OSSIFICANS PROGRESSIVA TREATMENT MARKET, BY END USER
14.1 OVERVIEW
14.2 HOSPITALS
14.3 SPECIALITY CLINICS
14.4 HOMECARE SETTING
14.5 ACADEMIC AND GOVERNMENT RESEARCH INSTITUTES
14.6 OTHERS
15 GLOBAL FIBRODYSPLASIA OSSIFICANS PROGRESSIVA TREATMENT MARKET, BY DISTRIBUTION CHANNEL
15.1 OVERVIEW
15.2 DIRECT TENDER
15.3 RETAIL SALES
15.3.1 HOSPITAL PHARMACY
15.3.2 RETAIL PHARMACY
15.3.3 ONLINE PHARMACY
16 GLOBAL FIBRODYSPLASIA OSSIFICANS PROGRESSIVA TREATMENT MARKET, BY REGION
GLOBAL FIBRODYSPLASIA OSSIFICANS PROGRESSIVA TREATMENT MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
16.1 NORTH AMERICA
16.1.1 U.S.
16.1.2 CANADA
16.1.3 MEXICO
16.2 EUROPE
16.2.1 GERMANY
16.2.2 U.K.
16.2.3 ITALY
16.2.4 FRANCE
16.2.5 SPAIN
16.2.6 RUSSIA
16.2.7 SWITZERLAND
16.2.8 TURKEY
16.2.9 BELGIUM
16.2.10 NETHERLANDS
16.2.11 DENMARK
16.2.12 SWEDEN
16.2.13 POLAND
16.2.14 NORWAY
16.2.15 FINLAND
16.2.16 REST OF EUROPE
16.3 ASIA-PACIFIC
16.3.1 JAPAN
16.3.2 CHINA
16.3.3 SOUTH KOREA
16.3.4 INDIA
16.3.5 SINGAPORE
16.3.6 THAILAND
16.3.7 INDONESIA
16.3.8 MALAYSIA
16.3.9 PHILIPPINES
16.3.10 AUSTRALIA
16.3.11 NEW ZEALAND
16.3.12 VIETNAM
16.3.13 TAIWAN
16.3.14 REST OF ASIA-PACIFIC
16.4 SOUTH AMERICA
16.4.1 BRAZIL
16.4.2 ARGENTINA
16.4.3 REST OF SOUTH AMERICA
16.5 MIDDLE EAST AND AFRICA
16.5.1 SOUTH AFRICA
16.5.2 EGYPT
16.5.3 BAHRAIN
16.5.4 UNITED ARAB EMIRATES
16.5.5 KUWAIT
16.5.6 OMAN
16.5.7 QATAR
16.5.8 SAUDI ARABIA
16.5.9 REST OF MIDDLE EAST AND AFRICA
16.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
17 GLOBAL FIBRODYSPLASIA OSSIFICANS PROGRESSIVA TREATMENT MARKET, COMPANY LANDSCAPE
17.1 COMPANY SHARE ANALYSIS: GLOBAL
17.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
17.3 COMPANY SHARE ANALYSIS: EUROPE
17.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
17.5 MERGERS & ACQUISITIONS
17.6 NEW PRODUCT DEVELOPMENT & APPROVALS
17.7 EXPANSIONS
17.8 REGULATORY CHANGES
17.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
18 GLOBAL FIBRODYSPLASIA OSSIFICANS PROGRESSIVA TREATMENT MARKET, COMPANY PROFILE
18.1 PFIZER INC.
18.1.1 COMPANY OVERVIEW
18.1.2 REVENUE ANALYSIS
18.1.3 GEOGRAPHIC PRESENCE
18.1.4 PRODUCT PORTFOLIO
18.1.5 RECENT DEVELOPMENTS
18.2 ABBVIE INC
18.2.1 COMPANY OVERVIEW
18.2.2 REVENUE ANALYSIS
18.2.3 GEOGRAPHIC PRESENCE
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPEMENTS
18.3 SANDOZ CAC
18.3.1 COMPANY OVERVIEW
18.3.2 REVENUE ANALYSIS
18.3.3 GEOGRAPHIC PRESENCE
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPEMENTS
18.4 AMNEAL PHARMACEUTICALS LLC.,
18.4.1 COMPANY OVERVIEW
18.4.2 REVENUE ANALYSIS
18.4.3 GEOGRAPHIC PRESENCE
18.4.4 PRODUCT PORTFOLIO
18.4.5 RECENT DEVELOPEMENTS
18.5 CAMBER PHARMACEUTICALS, INC.
18.5.1 COMPANY OVERVIEW
18.5.2 REVENUE ANALYSIS
18.5.3 GEOGRAPHIC PRESENCE
18.5.4 PRODUCT PORTFOLIO
18.5.5 RECENT DEVELOPEMENTS
18.6 MERCK & CO INC.
18.6.1 COMPANY OVERVIEW
18.6.2 REVENUE ANALYSIS
18.6.3 GEOGRAPHIC PRESENCE
18.6.4 PRODUCT PORTFOLIO
18.6.5 RECENT DEVELOPEMENTS
18.7 SUN PHARMACEUTICAL INDUSTRIES LTD.
18.7.1 COMPANY OVERVIEW
18.7.2 REVENUE ANALYSIS
18.7.3 GEOGRAPHIC PRESENCE
18.7.4 PRODUCT PORTFOLIO
18.7.5 RECENT DEVELOPEMENTS
18.8 TEVA PHARMACEUTICALS USA, INC.
18.8.1 COMPANY OVERVIEW
18.8.2 REVENUE ANALYSIS
18.8.3 GEOGRAPHIC PRESENCE
18.8.4 PRODUCT PORTFOLIO
18.8.5 RECENT DEVELOPEMENTS
18.9 OCEANIC PHARMACHEM PVT. LTD
18.9.1 COMPANY OVERVIEW
18.9.2 REVENUE ANALYSIS
18.9.3 GEOGRAPHIC PRESENCE
18.9.4 PRODUCT PORTFOLIO
18.9.5 RECENT DEVELOPEMENTS
18.1 DM PHARMA
18.10.1 COMPANY OVERVIEW
18.10.2 REVENUE ANALYSIS
18.10.3 GEOGRAPHIC PRESENCE
18.10.4 PRODUCT PORTFOLIO
18.10.5 RE
18.10.6 CENT DEVELOPEMENTS
18.11 PAR PHARMACEUTICAL
18.11.1 COMPANY OVERVIEW
18.11.2 REVENUE ANALYSIS
18.11.3 GEOGRAPHIC PRESENCE
18.11.4 PRODUCT PORTFOLIO
18.11.5 RECENT DEVELOPEMENTS
18.12 HORIZON THERAPEUTICS PLC
18.12.1 COMPANY OVERVIEW
18.12.2 REVENUE ANALYSIS
18.12.3 GEOGRAPHIC PRESENCE
18.12.4 PRODUCT PORTFOLIO
18.12.5 RECENT DEVELOPEMENTS
18.13 JUBILANT CADISTA PHARMACEUTICALS INC
18.13.1 COMPANY OVERVIEW
18.13.2 REVENUE ANALYSIS
18.13.3 GEOGRAPHIC PRESENCE
18.13.4 PRODUCT PORTFOLIO
18.13.5 RECENT DEVELOPEMENTS
18.14 PRUDENCE PHARMA CHEM -
18.14.1 COMPANY OVERVIEW
18.14.2 REVENUE ANALYSIS
18.14.3 GEOGRAPHIC PRESENCE
18.14.4 PRODUCT PORTFOLIO
18.14.5 RECENT DEVELOPEMENTS
18.15 THERMO FISHER SCIENTIFIC
18.15.1 COMPANY OVERVIEW
18.15.2 REVENUE ANALYSIS
18.15.3 GEOGRAPHIC PRESENCE
18.15.4 PRODUCT PORTFOLIO
18.15.5 RECENT DEVELOPEMENTS
18.16 VINEET LABORATORIES LIMITED,
18.16.1 COMPANY OVERVIEW
18.16.2 REVENUE ANALYSIS
18.16.3 GEOGRAPHIC PRESENCE
18.16.4 PRODUCT PORTFOLIO
18.16.5 RECENT DEVELOPEMENTS
18.17 LANNETT
18.17.1 COMPANY OVERVIEW
18.17.2 REVENUE ANALYSIS
18.17.3 GEOGRAPHIC PRESENCE
18.17.4 PRODUCT PORTFOLIO
18.17.5 RECENT DEVELOPEMENTS
18.18 ALEMBIC PHARMACEUTICALS LIMITED
18.18.1 COMPANY OVERVIEW
18.18.2 REVENUE ANALYSIS
18.18.3 GEOGRAPHIC PRESENCE
18.18.4 PRODUCT PORTFOLIO
18.18.5 RECENT DEVELOPEMENTS
18.19 APOTEX INC.
18.19.1 COMPANY OVERVIEW
18.19.2 REVENUE ANALYSIS
18.19.3 GEOGRAPHIC PRESENCE
18.19.4 PRODUCT PORTFOLIO
18.19.5 RECENT DEVELOPEMENTS
18.2 IPSEN PHARMA
18.20.1 COMPANY OVERVIEW
18.20.2 REVENUE ANALYSIS
18.20.3 GEOGRAPHIC PRESENCE
18.20.4 PRODUCT PORTFOLIO
18.20.5 RECENT DEVELOPEMENTS
18.21 REGENERON PHARMACEUTICALS INC
18.21.1 COMPANY OVERVIEW
18.21.2 REVENUE ANALYSIS
18.21.3 GEOGRAPHIC PRESENCE
18.21.4 PRODUCT PORTFOLIO
18.21.5 RECENT DEVELOPEMENTS
18.22 INCYTE.
18.22.1 COMPANY OVERVIEW
18.22.2 REVENUE ANALYSIS
18.22.3 GEOGRAPHIC PRESENCE
18.22.4 PRODUCT PORTFOLIO
18.22.5 RECENT DEVELOPEMENTS
18.23 BIOCRYST PHARMACEUTICALS, INC
18.23.1 COMPANY OVERVIEW
18.23.2 REVENUE ANALYSIS
18.23.3 GEOGRAPHIC PRESENCE
18.23.4 PRODUCT PORTFOLIO
18.23.5 RECENT DEVELOPEMENTS
18.24 DAIICHI SANKYO COMPANY, LIMITED
18.24.1 COMPANY OVERVIEW
18.24.2 REVENUE ANALYSIS
18.24.3 GEOGRAPHIC PRESENCE
18.24.4 PRODUCT PORTFOLIO
18.24.5 RECENT DEVELOPEMENTS
18.25 KEROS THERAPEUTICS
18.25.1 COMPANY OVERVIEW
18.25.2 REVENUE ANALYSIS
18.25.3 GEOGRAPHIC PRESENCE
18.25.4 PRODUCT PORTFOLIO
18.25.5 RECENT DEVELOPEMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
19 CONCLUSION
20 QUESTIONNAIRE
21 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












