Global Immune Repertoire Sequencing Market
Market Size in USD Million
CAGR :
% 
 USD
149.54 Million
USD
255.98 Million
2021
2029
USD
149.54 Million
USD
255.98 Million
2021
2029
| 2022 –2029 | |
| USD 149.54 Million | |
| USD 255.98 Million | |
|
|
|
|
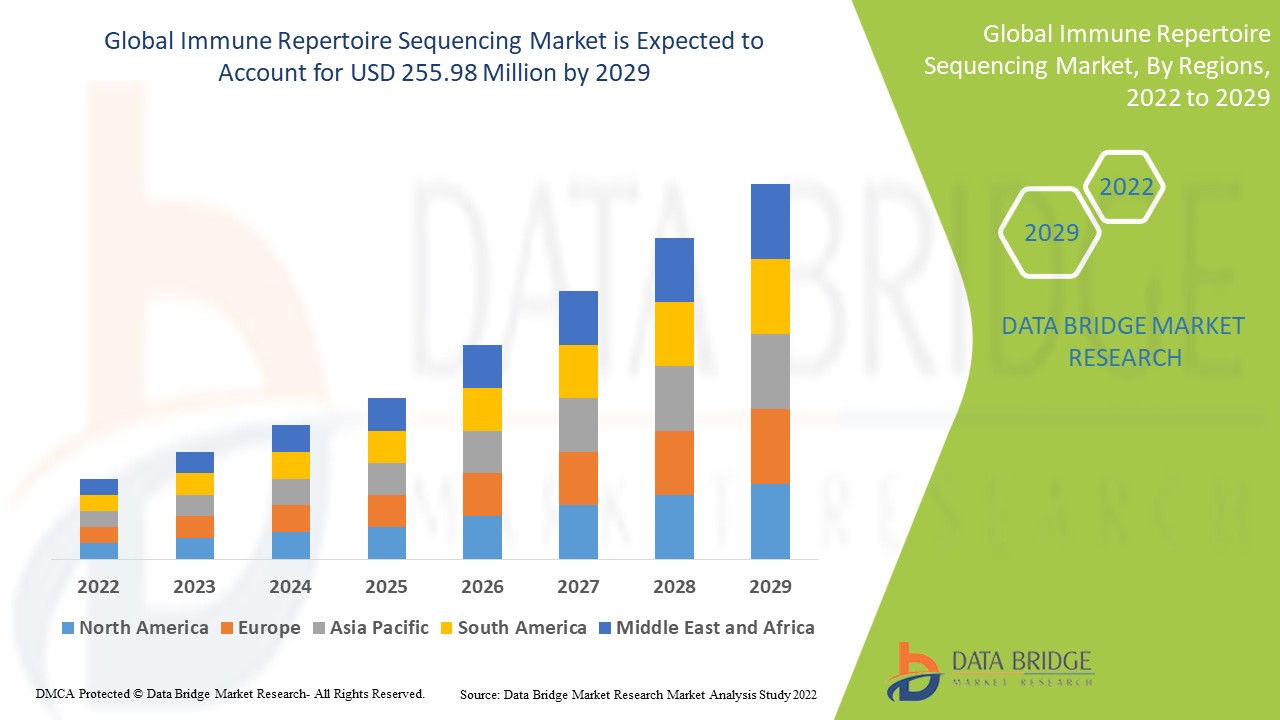
Global Immune Repertoire Sequencing Market Analysis and Size
Immune repertoire sequencing is widely used in biomarker discovery to outgrow the success rate and cost-effectiveness of rational drug development. For instance, the Center for Commercialization of Cancer Immunotherapy (C3i) in November 2017 started providing T cell receptor-beta sequencing facilities in its Biomarker and Diagnostic unit in Canada. Rising demand for personalized medicine is expected to help the immune repertoire sequencing market grow, as personalized medicine uses the human genetic profile to make decisions regarding disease diagnosis, prevention, and treatment.
Data Bridge Market Research analyses a growth in the global immune repertoire sequencing market in the forecast period 2022-2029. The expected CAGR of immune repertoire sequencing market tends to be around 6.95% in the mentioned forecast period. The market was valued at USD 149.54 million in 2021, and it would grow upto USD 255.98 million by 2029. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Global Immune Repertoire Sequencing Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2014 - 2019) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product Type (Assay Kits and Instruments), Application (Cancer Immunotherapy, Biomarker Discovery, Autoimmune Diseases, Vaccine Development and Efficacy, Transplant Rejection and Tolerance, Infectious Diseases and Other), End-User (Diagnostics Labs, Biotechnology and Pharmaceuticals Companies, Academic and Research Institutes and Others) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America. |
|
Market Players Covered |
Illumina Inc. (U.S.), Thermo Fisher Scientific (U.S.), PacBio (U.S.), CD Genomics (U.S.), Agilent Technologies, Inc (U.S.).,F. Hoffmann-La Roche Ltd (Switzerland), BGI Group Guangdong (Japan), Takara Bio Inc. (Japan), Adaptive Biotechnologies (U.S.), Atreca, Inc. (U.S.)., iRepertoire, Inc. (U.S.) |
|
Market Opportunities |
|
Market Definition
Human adaptive immune system protection is interfered with by receptors on the surface of T and B cells known as T-cell receptors and B-cell receptors. Next-generation sequencing has enabled the development of an emerging technology known as immune repertoire sequencing for probing complimentary, which works to determine the regions of these receptors. Single-cell approaches are most likely to dominate immune repertoire sequencing because they provide high-dimensional and in-depth information on smaller populations of interest, including antigen-binding cells. Thus, this is of great importance to the emerging market.
Global Immune Repertoire Sequencing Market Dynamics
Drivers
- Increasing Research and Development
In the near future, increasing research and development and introducing novel products by key players are projected to be the primary drivers of global immune repertoire sequencing market growth. For instance, during the American Society of Hematology in 2016, ArcherDX, Inc. increased its product line by introducing Archer Immunoverse immune repertoire sequencing tests (ASH). Archer Immunoverse B Cell Receptor (BCR) tests were introduced in 2017 by ArcherDX, Inc. to define the human B cell repertoire.
- Increased Government Support
Increase in support from the government for varied pharmacogenomics-based drug recovery is the major driver of the global immune repertoire sequencing market. In addition to this, the rapid rise in various therapeutic areas such as infectious diseases, cardiology, oncology, and pain management prompts the growth of the global immune repertoire sequencing market.
Opportunities
- Increased Partnerships Promote High Market Growth
Another significant factor expected to contribute to the growth of the global immune repertoire sequencing market during the forecast period is manufacturer agreements and partnerships. For instance, ArcherDX and Ambry Genetics formed a collaboration in March 2018 to create new and breakthrough immunotherapies.
- Increasing Demand For Personalized Medicine
The rise in demand for personalized medicine will positively influence the growth of the global immune repertoire sequencing market. In addition, nurturing the role of next-generation immune repertoire sequencing for personalized immune modulation will surely support the global immune repertoire sequencing market growth.
Restraints/Challenges
- Lack of skilled professionals
The lack of qualified personnel to perform immune repertoire sequencing techniques developed in specialized laboratories could curb the growth of the immune repertoire sequencing market over a forecast period.
- Accuracy Issues and Biased Techniques
Differentiating biological changes from errors and bias introduced in different phases is a challenge in immune repertoire sequencing. Due to insufficient scalability and sensitivity and limitations in supplying sufficient and reliable data, traditional and novel bioinformatics techniques such as high throughput screening, Haystack Heuristic, and others fall short. Furthermore, discrepancies in data collection and processing in different laboratories, as well as obstacles in immune repertoire sequencing systems, are some of the significant reasons that could limit the immune repertoire sequencing market's growth.
This immune repertoire sequencing market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the immune repertoire sequencing market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on Global Immune Repertoire Sequencing Market
The COVID-19 has left an unprecedented global public health crisis that has impacted practically every business. Its long-term repercussions are expected to influence industry growth during the forecast period. Our ongoing research is enhancing our research approach to guarantee that fundamental COVID-19 concerns and potential solutions are included.
Due to undermining clinical studies, insufficient supply chain management and raw material storage, and patient noncompliance, the COVID-19 pandemic has substantially impacted the worldwide immune repertoire sequencing market. Furthermore, the worldwide immune repertoire sequencing market has been significantly impacted by governments' reduced financing as a result of the pandemic. However, in the post-pandemic situation, the market is slowly improving as the funds are returning and it is expected to flourish at a much higher rate.
Global Immune Repertoire Sequencing Market Scope
The immune repertoire sequencing market is segmented on the basis of product type, application and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product Type
- Assay Kits
- Instruments
Application
- Cancer Immunotherapy
- Biomarker Discovery
- Autoimmune Diseases
- Vaccine Development and Efficacy
- Transplant Rejection and Tolerance
- Infectious Diseases
- Other
End-User
- Diagnostics Labs
- Biotechnology and Pharmaceuticals Companies
- Academic and Research Institutes
- Others
Immune Repertoire Sequencing Market Regional Analysis/Insights
The immune repertoire sequencing market is analysed and market size insights and trends are provided by country, product type, application and end-user as referenced above.
The major countries covered in the immune repertoire sequencing market report are the U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the immune repertoire sequencing market due to rapid increase in the demand for immunotherapy for cancer and other infectious diseases.
Asia-Pacific is expected to expand at a significant growth rate during the forecast period of 2022 to 2029 due to international collaborations for immune repertoire sequencing and developments.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Global Immune Repertoire Sequencing Market Share Analysis
The immune repertoire sequencing market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to immune repertoire sequencing market
Key players operating in the immune repertoire sequencing market include:
- Illumina Inc. (U.S.)
- Thermo Fisher Scientific (U.S.)
- PacBio (U.S.)
- CD Genomics (U.S.)
- Agilent Technologies, Inc (U.S.).
- F. Hoffmann-La Roche Ltd (Switzerland)
- BGI Group Guangdong (Japan)
- Takara Bio, Inc. (Japan)
- Adaptive Biotechnologies (U.S.)
- Juno Therapeutics (U.S.)
- Atreca, Inc. (U.S.)
- irepertoire, Inc. (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL IMMUNE REPERTOIRE SEQUENCING MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL IMMUNE REPERTOIRE SEQUENCING MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL IMMUNE REPERTOIRE SEQUENCING MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 REGULATORY SCENARIO
6 PREMIUM INSIGHTS
6.1 PESTLE ANALYSIS
6.2 PORTER’S FIVE FORCES
7 INDUSTRY INSIGHTS
8 GLOBAL IMMUNE REPERTOIRE SEQUENCING MARKET, BY PRODUCT & SERVICES
8.1 OVERVIEW
8.2 KITS AND CONSUMBALES
8.2.1 KITS
8.2.1.1. TCR KITS
8.2.1.1.1. HUMAN TCR KITS
8.2.1.1.1.1 MARKET VALUE (USD MILLION)
8.2.1.1.1.2 MARKET VOLUME (UNITS)
8.2.1.1.1.3 AVERAGE SELLING PRICE (USD)
8.2.1.1.2. MOUSE TCR KITS
8.2.1.1.2.1 MARKET VALUE (USD MILLION)
8.2.1.1.2.2 MARKET VOLUME (UNITS)
8.2.1.1.2.3 AVERAGE SELLING PRICE (USD)
8.2.1.2. BCR KITS
8.2.1.2.1. HUMAN BCR KITS
8.2.1.2.1.1 MARKET VALUE (USD MILLION)
8.2.1.2.1.2 MARKET VOLUME (UNITS)
8.2.1.2.1.3 AVERAGE SELLING PRICE (USD)
8.2.1.2.2. MOUSE BCR KITS
8.2.1.2.2.1 MARKET VALUE (USD MILLION)
8.2.1.2.2.2 MARKET VOLUME (UNITS)
8.2.1.2.2.3 AVERAGE SELLING PRICE (USD)
8.2.2 PANEL AND ASSAYS
8.2.2.1. TRANSCRIPTOME PANEL
8.2.2.1.1. MARKET VALUE (USD MILLION)
8.2.2.1.2. MARKET VOLUME (UNITS)
8.2.2.1.3. AVERAGE SELLING PRICE (USD)
8.2.2.2. PROTEIN ASSAYS
8.2.2.2.1. MARKET VALUE (USD MILLION)
8.2.2.2.2. MARKET VOLUME (UNITS)
8.2.2.2.3. AVERAGE SELLING PRICE (USD)
8.2.2.3. PROTEIN STAIN REAGENT KITS
8.2.2.3.1. MARKET VALUE (USD MILLION)
8.2.2.3.2. MARKET VOLUME (UNITS)
8.2.2.3.3. AVERAGE SELLING PRICE (USD)
8.2.2.4. OTHERS
8.3 INSTRUMENTS
8.3.1 IMMUNE REPERTOIRE ANALYZER
8.3.1.1. MARKET VALUE (USD MILLION)
8.3.1.2. MARKET VOLUME (UNITS)
8.3.1.3. AVERAGE SELLING PRICE (USD)
8.3.2 SPATIAL PROFILER
8.3.2.1. MARKET VALUE (USD MILLION)
8.3.2.2. MARKET VOLUME (UNITS)
8.3.2.3. AVERAGE SELLING PRICE (USD)
8.3.3 NGS PLATFORMS
8.3.3.1. MARKET VALUE (USD MILLION)
8.3.3.2. MARKET VOLUME (UNITS)
8.3.3.3. AVERAGE SELLING PRICE (USD)
8.3.4 PCR
8.3.4.1. MARKET VALUE (USD MILLION)
8.3.4.2. MARKET VOLUME (UNITS)
8.3.4.3. AVERAGE SELLING PRICE (USD)
8.3.5 OTHERS
8.4 SOFTWARE
8.4.1 TCR ANALYSIS SOLUTIONS
8.4.1.1. MARKET VALUE (USD MILLION)
8.4.1.2. MARKET VOLUME (UNITS)
8.4.1.3. AVERAGE SELLING PRICE (USD)
8.4.2 BCR ANALYSIS SOLUTIONS
8.4.2.1. MARKET VALUE (USD MILLION)
8.4.2.2. MARKET VOLUME (UNITS)
8.4.2.3. AVERAGE SELLING PRICE (USD)
8.4.3 TCR & BCR ANALYSIS SOLUTIONS
8.4.3.1. MARKET VALUE (USD MILLION)
8.4.3.2. MARKET VOLUME (UNITS)
8.4.3.3. AVERAGE SELLING PRICE (USD)
8.5 SERVICES
8.5.1 PRE-SEQUENCING SERVICES
8.5.2 SEQUENCING SERVICES
8.5.3 DATA ANALYSIS SERVICES
8.5.4 OTHER
9 GLOBAL IMMUNE REPERTOIRE SEQUENCING MARKET, BY TECHNOLOGY
9.1 OVERVIEW
9.2 SPATIAL BIOLOGY
9.3 NGS TECHNOLOGY
9.4 5’ RAPID AMPLIFICATION OF CDNA ENDS (RACE) TECHNOLOGY
9.5 AMPLICON RESCUED MULTIPLEX PCR (ARM PCR)
9.6 DIMER AVOIDED MULTIPLEX PCR (DAM PCR)
9.7 OTHER
10 GLOBAL IMMUNE REPERTOIRE SEQUENCING MARKET, BY APPLICATION
10.1 OVERVIEW
10.2 CLINICAL
10.2.1 CANCER IMMUNOTHERAPY
10.2.1.1. ASSAY KITS
10.2.1.2. INSTRUMENTS
10.2.1.3. SOFTWARE
10.2.1.4. SERVICES
10.2.2 AUTOIMMUNE DISEASES
10.2.2.1. ASSAY KITS
10.2.2.2. INSTRUMENTS
10.2.2.3. SOFTWARE
10.2.2.4. SERVICES
10.2.3 INFECTIOUS DISEASES
10.2.3.1. ASSAY KITS
10.2.3.2. INSTRUMENTS
10.2.3.3. SOFTWARE
10.2.3.4. SERVICES
10.3 RESEARCH
10.3.1 BIOMARKER DISCOVERY
10.3.1.1. ASSAY KITS
10.3.1.2. INSTRUMENTS
10.3.1.3. SOFTWARE
10.3.1.4. SERVICES
10.3.2 VACCINE DEVELOPMENT AND EFFICACY
10.3.2.1. ASSAY KITS
10.3.2.2. INSTRUMENTS
10.3.2.3. SOFTWARE
10.3.2.4. SERVICES
10.3.3 TRANSPLANT REJECTION AND TOLERANCE
10.3.3.1. ASSAY KITS
10.3.3.2. INSTRUMENTS
10.3.3.3. SOFTWARE
10.3.3.4. SERVICES
10.3.4 ANTIBODY DISCOVERY
10.3.4.1. ASSAY KITS
10.3.4.2. INSTRUMENTS
10.3.4.3. SOFTWARE
10.3.4.4. SERVICES
10.3.5 IMMUNODEFICIENCY IDENTIFICATION
10.3.5.1. ASSAY KITS
10.3.5.2. INSTRUMENTS
10.3.5.3. SOFTWARE
10.3.5.4. SERVICES
10.4 OTHER
11 GLOBAL IMMUNE REPERTOIRE SEQUENCING MARKET, BY END USER
11.1 OVERVIEW
11.2 MOLECULAR DIAGNOSTIC LABS
11.3 BIOTECHNOLOGY AND PHARMACEUTICAL COMPANIES
11.4 ACADEMIC AND RESEARCH INSTITUTES
11.5 CONTRACT RESEARCH ORGANIZATION (CRO)
11.6 CONTRACT DEVELOPMENT & MANUFACTURING ORGANIZATION (CDMO)
11.7 OTHERS
12 GLOBAL IMMUNE REPERTOIRE SEQUENCING MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDER
12.3 RETAIL SALES
12.4 ONLINE SALES
12.5 OTHER
13 GLOBAL IMMUNE REPERTOIRE SEQUENCING MARKET, COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
13.2 COMPANY SHARE ANALYSIS: EUROPE
13.3 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
13.4 MERGERS & ACQUISITIONS
13.5 NEW PRODUCT DEVELOPMENT & APPROVALS
13.6 EXPANSIONS
13.7 REGULATORY CHANGES
13.8 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
14 GLOBAL IMMUNE REPERTOIRE SEQUENCING MARKET, BY REGION
GLOBAL IMMUNE REPERTOIRE SEQUENCING MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
14.1 NORTH AMERICA
14.1.1 U.S.
14.1.2 CANADA
14.1.3 MEXICO
14.2 EUROPE
14.2.1 GERMANY
14.2.2 U.K.
14.2.3 ITALY
14.2.4 FRANCE
14.2.5 SPAIN
14.2.6 RUSSIA
14.2.7 SWITZERLAND
14.2.8 TURKEY
14.2.9 BELGIUM
14.2.10 NETHERLANDS
14.2.11 DENMARK
14.2.12 SWEDEN
14.2.13 POLAND
14.2.14 NORWAY
14.2.15 FINLAND
14.2.16 REST OF EUROPE
14.3 ASIA-PACIFIC
14.3.1 JAPAN
14.3.2 CHINA
14.3.3 SOUTH KOREA
14.3.4 INDIA
14.3.5 SINGAPORE
14.3.6 THAILAND
14.3.7 INDONESIA
14.3.8 MALAYSIA
14.3.9 PHILIPPINES
14.3.10 AUSTRALIA
14.3.11 NEW ZEALAND
14.3.12 VIETNAM
14.3.13 TAIWAN
14.3.14 REST OF ASIA-PACIFIC
14.4 SOUTH AMERICA
14.4.1 BRAZIL
14.4.2 ARGENTINA
14.4.3 REST OF SOUTH AMERICA
14.5 MIDDLE EAST AND AFRICA
14.5.1 SOUTH AFRICA
14.5.2 EGYPT
14.5.3 BAHRAIN
14.5.4 UNITED ARAB EMIRATES
14.5.5 KUWAIT
14.5.6 OMAN
14.5.7 QATAR
14.5.8 SAUDI ARABIA
14.5.9 REST OF MEA
14.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
15 GLOBAL IMMUNE REPERTOIRE SEQUENCING MARKET, SWOT AND DBMR ANALYSIS
16 GLOBAL IMMUNE REPERTOIRE SEQUENCING MARKET, COMPANY PROFILE
16.1 ILLUMINA INC.
16.1.1 COMPANY OVERVIEW
16.1.2 REVENUE ANALYSIS
16.1.3 GEOGRAPHIC PRESENCE
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPEMENTS
16.2 THERMO FISHER SCIENTIFIC
16.2.1 COMPANY OVERVIEW
16.2.2 REVENUE ANALYSIS
16.2.3 GEOGRAPHIC PRESENCE
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPEMENTS
16.3 PACBIO
16.3.1 COMPANY OVERVIEW
16.3.2 REVENUE ANALYSIS
16.3.3 GEOGRAPHIC PRESENCE
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT DEVELOPEMENTS
16.4 CD GENOMICS
16.4.1 COMPANY OVERVIEW
16.4.2 REVENUE ANALYSIS
16.4.3 GEOGRAPHIC PRESENCE
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT DEVELOPEMENTS
16.5 AGILENT TECHNOLOGIES, INC
16.5.1 COMPANY OVERVIEW
16.5.2 REVENUE ANALYSIS
16.5.3 GEOGRAPHIC PRESENCE
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPEMENTS
16.6 F. HOFFMANN-LA ROCHE LTD
16.6.1 COMPANY OVERVIEW
16.6.2 REVENUE ANALYSIS
16.6.3 GEOGRAPHIC PRESENCE
16.6.4 PRODUCT PORTFOLIO
16.6.5 RECENT DEVELOPEMENTS
16.7 BGI
16.7.1 COMPANY OVERVIEW
16.7.2 REVENUE ANALYSIS
16.7.3 GEOGRAPHIC PRESENCE
16.7.4 PRODUCT PORTFOLIO
16.7.5 RECENT DEVELOPEMENTS
16.8 TAKARA BIO, INC.
16.8.1 COMPANY OVERVIEW
16.8.2 REVENUE ANALYSIS
16.8.3 GEOGRAPHIC PRESENCE
16.8.4 PRODUCT PORTFOLIO
16.8.5 RECENT DEVELOPEMENTS
16.9 ADAPTIVE BIOTECHNOLOGIES
16.9.1 COMPANY OVERVIEW
16.9.2 REVENUE ANALYSIS
16.9.3 GEOGRAPHIC PRESENCE
16.9.4 PRODUCT PORTFOLIO
16.9.5 RECENT DEVELOPEMENTS
16.1 JUNO THERAPEUTICS
16.10.1 COMPANY OVERVIEW
16.10.2 REVENUE ANALYSIS
16.10.3 GEOGRAPHIC PRESENCE
16.10.4 PRODUCT PORTFOLIO
16.10.5 RECENT DEVELOPEMENTS
16.11 ATRECA, INC.
16.11.1 COMPANY OVERVIEW
16.11.2 REVENUE ANALYSIS
16.11.3 GEOGRAPHIC PRESENCE
16.11.4 PRODUCT PORTFOLIO
16.11.5 RECENT DEVELOPEMENTS
16.12 IREPERTOIRE, INC
16.12.1 COMPANY OVERVIEW
16.12.2 REVENUE ANALYSIS
16.12.3 GEOGRAPHIC PRESENCE
16.12.4 PRODUCT PORTFOLIO
16.12.5 RECENT DEVELOPEMENTS
16.13 QIAGEN
16.13.1 COMPANY OVERVIEW
16.13.2 REVENUE ANALYSIS
16.13.3 GEOGRAPHIC PRESENCE
16.13.4 PRODUCT PORTFOLIO
16.13.5 RECENT DEVELOPEMENTS
16.14 TAKARA BIO INC
16.14.1 COMPANY OVERVIEW
16.14.2 REVENUE ANALYSIS
16.14.3 GEOGRAPHIC PRESENCE
16.14.4 PRODUCT PORTFOLIO
16.14.5 RECENT DEVELOPEMENTS
16.15 OXFORD NANOPORE TECHNOLOGIES PLC
16.15.1 COMPANY OVERVIEW
16.15.2 REVENUE ANALYSIS
16.15.3 GEOGRAPHIC PRESENCE
16.15.4 PRODUCT PORTFOLIO
16.15.5 RECENT DEVELOPEMENTS
16.16 SYNBIO TECHNOLOGIES
16.16.1 COMPANY OVERVIEW
16.16.2 REVENUE ANALYSIS
16.16.3 GEOGRAPHIC PRESENCE
16.16.4 PRODUCT PORTFOLIO
16.16.5 RECENT DEVELOPEMENTS
16.17 NEW ENGLAND BIOLABS.
16.17.1 COMPANY OVERVIEW
16.17.2 REVENUE ANALYSIS
16.17.3 GEOGRAPHIC PRESENCE
16.17.4 PRODUCT PORTFOLIO
16.17.5 RECENT DEVELOPEMENTS
16.18 MEDGENOME
16.18.1 COMPANY OVERVIEW
16.18.2 REVENUE ANALYSIS
16.18.3 GEOGRAPHIC PRESENCE
16.18.4 PRODUCT PORTFOLIO
16.18.5 RECENT DEVELOPEMENTS
16.19 ARCHER( INTEGRATED DNA TECHNOLOGIES, INC.)
16.19.1 COMPANY OVERVIEW
16.19.2 REVENUE ANALYSIS
16.19.3 GEOGRAPHIC PRESENCE
16.19.4 PRODUCT PORTFOLIO
16.19.5 RECENT DEVELOPEMENTS
16.2 ALACRIS THERANOSTICS GMBH
16.20.1 COMPANY OVERVIEW
16.20.2 REVENUE ANALYSIS
16.20.3 GEOGRAPHIC PRESENCE
16.20.4 PRODUCT PORTFOLIO
16.20.5 RECENT DEVELOPEMENTS
16.21 EXPLOREA S.R.O.
16.21.1 COMPANY OVERVIEW
16.21.2 REVENUE ANALYSIS
16.21.3 GEOGRAPHIC PRESENCE
16.21.4 PRODUCT PORTFOLIO
16.21.5 RECENT DEVELOPEMENTS
16.22 DIAGNÓSTICA LONGWOOD SL
16.22.1 COMPANY OVERVIEW
16.22.2 REVENUE ANALYSIS
16.22.3 GEOGRAPHIC PRESENCE
16.22.4 PRODUCT PORTFOLIO
16.22.5 RECENT DEVELOPEMENTS
16.23 GENEWERK
16.23.1 COMPANY OVERVIEW
16.23.2 REVENUE ANALYSIS
16.23.3 GEOGRAPHIC PRESENCE
16.23.4 PRODUCT PORTFOLIO
16.23.5 RECENT DEVELOPEMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST RELATED REPORTS
17 RELATED REPORTS
18 QUESTIONNAIRE
19 ABOUT DATA BRIDGE MARKET RESEARCH
20 MARKET SEGMENTATION

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.













