Global Isothermal Nucleic Acid Amplification Tests Market
Market Size in USD Billion
CAGR :
% 
 USD
2.40 Billion
USD
5.90 Billion
2021
2029
USD
2.40 Billion
USD
5.90 Billion
2021
2029
| 2022 –2029 | |
| USD 2.40 Billion | |
| USD 5.90 Billion | |
|
|
|
|
Isothermal Nucleic Acid Amplification Tests Market Analysis and Size
According to the Centers for Disease Control and Prevention (CDC), 81 million Americans had various chronic ailments in 2020, making up about 157 million people overall. Because of factors such a large population base, lax regulatory environment, and strong need for affordable and diagnostics, the Asia Pacific is predicted to grow during the forecast period.
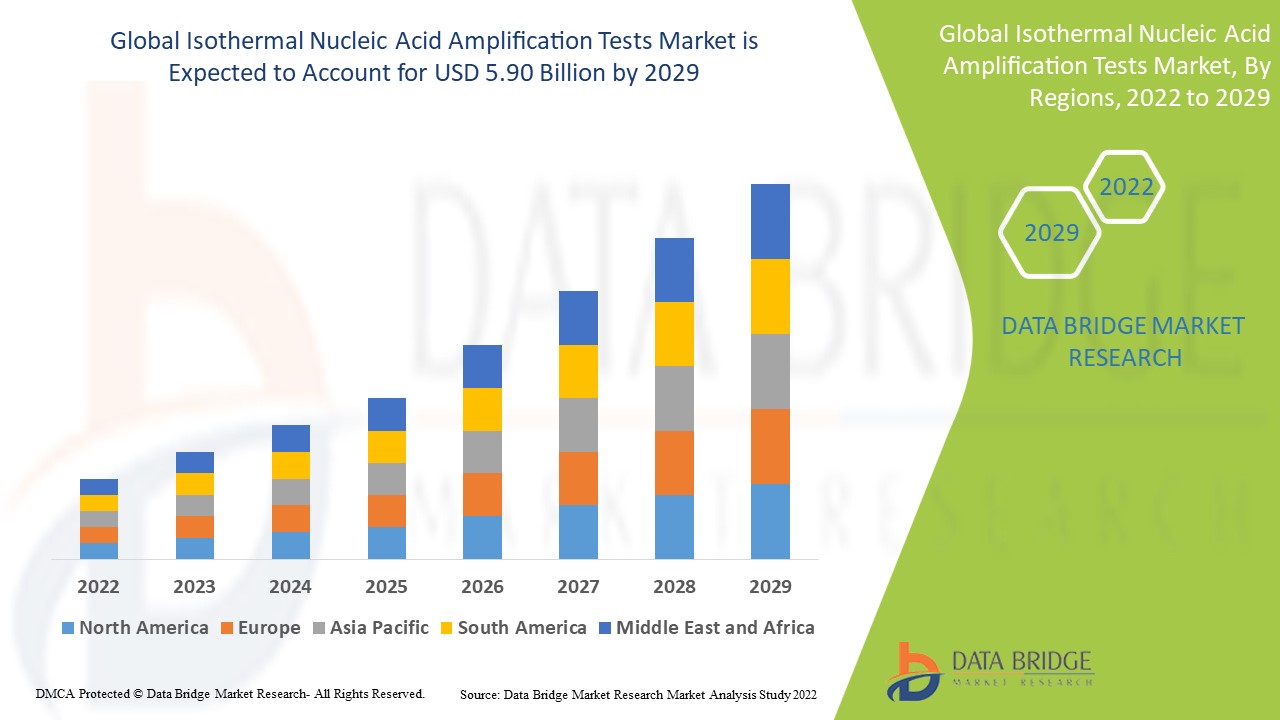
Data Bridge Market Research analyses that the isothermal nucleic acid amplification tests market which was USD 2.4 billion in 2021, would rocket up to USD 5.90 billion by 2029, and is expected to undergo a CAGR of 11.90% during the forecast period 2022 to 2029. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Isothermal Nucleic Acid Amplification Tests Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2014 - 2019) |
|
Quantitative Units |
Revenue in USD Billion, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product (Assay and Kit, System), Application (Infectious disease [HIV, Influenza], Blood Screening), Type (LAMP, SDA, NASBA, HAD, NEAR, TMA, SPIA, Others), Disease Indication (Coronary angioplasty, Venous angioplasty, Carotid angioplasty, Renal artery angioplasty, Peripheral angioplasty), End User (Hospitals, Research Laboratories, Others) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Siemens (Germany), Hologic, Inc. (U.S.), Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (China), Abbott (U.S.), BD (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), Thermo Fisher Scientific Inc. (U.S.), Koninklijke Philips N.V. (Netherlands), NeuroLogica Corp. (U.S.), Shimadzu Medical (India) pvt. Ltd. (Japan), GENERAL ELECTRIC (U.S.), Quest Diagnostics Incorporated (U.S.), Sysmex India Pvt. Ltd. (Japan), Hitachi, Ltd. (Japan), Canon Inc. (Japan), FUJIFILM Holdings Corporation (U.K.) |
|
Market Opportunities |
|
Market Definition
In molecular biology and recombinant DNA technologies, nucleic acids are detected and identified using the isothermal nucleic acid amplification technology (INAAT). It is frequently employed to amplify nucleic acids while maintaining a steady temperature, doing away with the requirement for thermocycler apparatus. It is mostly used for RNA, DNA, cells, proteins, tiny molecules, and ions to ensure the quick, sensitive, and precise diagnosis of genetic, hereditary, and infectious disorders.
Global Isothermal Nucleic Acid Amplification Tests Market Dynamics
Drivers
- Rising number of blood donations
The cost-benefits of INAAT and the rising number of blood donations and transfusions will spur market expansion. In the forecast period, various opportunities will emerge for the market for isothermal nucleic acid amplification tests due to growing opportunities in emerging markets and technological advancement and optimization.
- Rise in infectious diseases
Infectious diseases like H. influenzae, S. pneumonia (respiratory tract infections), N. gonorrhoeae, C. Trachomatis (genital infections), and TB are expected to become more prevalent in the coming years, which will spur innovation in isothermal nucleic acid amplification technology (INAAT) and propel the market.
Opportunities
The market for isothermal nucleic acid amplification assays is expanding as a result of more R&D projects being undertaken. This would present advantageous chances for the market expansion of isothermal nucleic acid amplification testing. Moreover, increase in the number of emerging markets and new product launches will further provide beneficial opportunities for the isothermal nucleic acid amplification tests market growth during the forecast period.
Restraints/Challenges
On the other hand, the widespread use of and dependence on PCR will obstruct the market's growth rate. The dearth of skilled professionals and lack of healthcare infrastructure in developing economies will challenge the isothermal nucleic acid amplification tests market.
This isothermal nucleic acid amplification tests market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the isothermal nucleic acid amplification tests market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on Isothermal Nucleic Acid Amplification Tests Market
COVID-19 had a positively impact on the market. While the in vitro diagnosis (IVD) field of study and industrial market flourished during and after the COVID-19 pandemic, the invention of isothermal nucleic acid amplification test (INAAT) based quick diagnosis was largely motivated by a need for problem-solving on a worldwide scale. The major performance metrics and cost-effectiveness of enzyme-assisted and enzyme-free methods were compared using the characteristics that make INAAT systems suitable for making diagnosis-relevant judgments. The methodology design and flexibility of the nucleic acid amplification reactions that can result in thresholding signal amplifications employing INAAT reagents, as well as the potential use with quick test platform/device integration.
Recent Development
- In April 2019, New freeze-dried lyophilized single-reaction reagent Qscript lyo 1-step was introduced by QuantaBio. Dry, consistent, simple, and superior 1-step RT-qPCR can be performed with Qscript Lyo (quantitative reverse transcription polymerase chain reaction). It is a one-step RT-qPCR that is extremely sensitive and repeatable. A thermostable hot-start polymerase is present in the reagent.
Global Isothermal Nucleic Acid Amplification Tests Market Scope
The isothermal nucleic acid amplification tests market is segmented on the basis of product, type, disease indication, application and end user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product
- Assay and Kit
- System
Application
- Infectious disease [HIV, Influenza]
- Blood Screening
Type
- LAMP
- SDA
- NASBA
- HAD
- NEAR
- TMA
- SPIA
- Others
Disease Indication
- Coronary angioplasty
- Venous angioplasty
- Carotid angioplasty
- Renal artery angioplasty
- Peripheral angioplasty
End User
- Hospitals
- Research Laboratories
- Others
Isothermal Nucleic Acid Amplification Tests Market Regional Analysis/Insights
The isothermal nucleic acid amplification tests market is analysed and market size insights and trends are provided by country, product, type, disease indication, application and end user as referenced above.
The countries covered in the isothermal nucleic acid amplification tests market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the isothermal nucleic acid amplification tests market owing to the presence of a well-established healthcare system, access to technologically advanced diagnostics and blood screening techniques, recommendations for blood screening, and a growing burden of infectious diseases.
Asia-Pacific is expected to grow at the highest growth rate in the forecast period of 2022 to 2029 due to the increasing research and development investments and rising expenditure on healthcare.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Isothermal Nucleic Acid Amplification Tests Market Share Analysis
The isothermal nucleic acid amplification tests market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to isothermal nucleic acid amplification tests market.
Some of the major players operating in the isothermal nucleic acid amplification tests market are:
- Siemens (Germany)
- Hologic, Inc. (U.S.)
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (China)
- Abbott (U.S.)
- BD (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Thermo Fisher Scientific Inc. (U.S.)
- Koninklijke Philips N.V. (Netherlands)
- NeuroLogica Corp. (U.S.)
- Shimadzu Medical (India) pvt. Ltd. (Japan)
- GENERAL ELECTRIC (U.S.)
- Quest Diagnostics Incorporated (U.S.)
- Sysmex India Pvt. Ltd. (Japan)
- Hitachi, Ltd. (Japan)
- Canon Inc. (Japan)
- FUJIFILM Holdings Corporation (U.K.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.













