Global Live Attenuated Vaccines Market
Market Size in USD Billion
CAGR :
% 
 USD
33.58 Billion
USD
84.96 Billion
2024
2032
USD
33.58 Billion
USD
84.96 Billion
2024
2032
| 2025 –2032 | |
| USD 33.58 Billion | |
| USD 84.96 Billion | |
|
|
|
|
Live Attenuated Vaccines Market Size
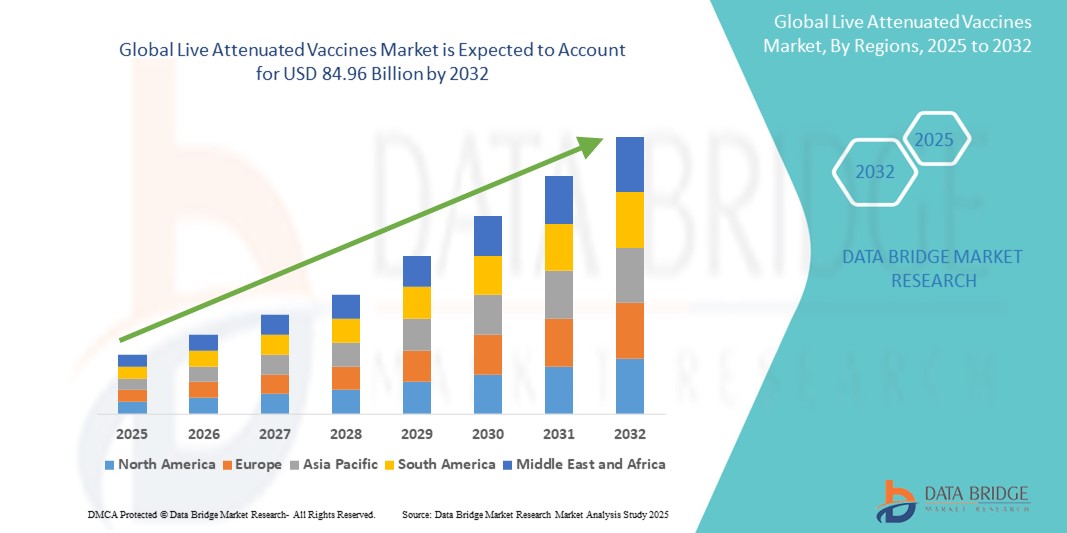
- The global live attenuated vaccines market size was valued at USD 33.58 billion in 2024 and is expected to reach USD 84.96 billion by 2032, at a CAGR of 12.30% during the forecast period
- The market growth is largely fueled by the rising prevalence of infectious diseases and increased focus on immunization programs by government and non-government health organizations, promoting the widespread use of live attenuated vaccines across both developed and developing countries
- Furthermore, growing awareness about long-lasting immunity offered by live attenuated vaccines, coupled with advancements in vaccine delivery technologies and strong support from global health initiatives such as GAVI and WHO, is accelerating the adoption of these vaccines, thereby significantly boosting the industry's growth
Live Attenuated Vaccines Market Analysis
- Live attenuated vaccines, which use a weakened form of the virus or bacteria to stimulate a strong and long-lasting immune response, are increasingly vital components of immunization programs in both pediatric and adult populations due to their high efficacy and ability to mimic natural infections
- The escalating demand for live attenuated vaccines is primarily fueled by the global rise in infectious diseases, expanded government vaccination initiatives, and growing public awareness about preventive healthcare and immunization
- North America dominated the live attenuated vaccines market with the largest revenue share of 40.01% in 2024, characterized by well-established healthcare infrastructure, high immunization coverage, and strong presence of major pharmaceutical companies, with the U.S. experiencing substantial growth in live attenuated vaccine administration across both public and private healthcare sectors
- Asia-Pacific is expected to be the fastest-growing region in the live attenuated vaccines market during the forecast period, projected to expand at a CAGR of 24.1%, driven by increasing birth rates, expanding government-funded immunization programs, and rising healthcare investments in countries like India and China
- Viral segment dominated the live attenuated vaccines market with a market share of 64.7% in 2024, owing to the high prevalence of viral infections such as measles, mumps, rubella, and polio, and the widespread adoption of viral-based live attenuated vaccines in global immunization programs
Report Scope and Live Attenuated Vaccines Market Segmentation
|
Attributes |
Live Attenuated Vaccines Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Live Attenuated Vaccines Market Trends
“Increased Global Immunization Efforts and Innovation in Vaccine Delivery”
- A significant and accelerating trend in the global live attenuated vaccines market is the rising emphasis on mass immunization programs led by governments and global health organizations such as WHO, GAVI, and UNICEF. These efforts are enhancing access to vaccines, especially in low- and middle-income countries, thus driving market expansion
- For instance, various national immunization schedules now include live attenuated vaccines for diseases like measles, mumps, rubella, rotavirus, and yellow fever, enabling wider distribution and adoption. This strategic push toward universal vaccination is playing a critical role in preventing outbreaks of vaccine-preventable diseases
- Innovation in vaccine storage, transportation, and needle-free delivery methods is further bolstering the reach and acceptance of live attenuated vaccines. Companies are developing thermostable formulations that require less stringent cold chain logistics, making it easier to distribute vaccines to remote or underdeveloped regions
- Moreover, partnerships between governments, NGOs, and pharmaceutical companies are accelerating clinical trials and approvals of newer live attenuated vaccines for emerging infectious diseases, improving global preparedness against future pandemics
- The increasing awareness of the long-term health benefits of early vaccination, combined with growing investments in public health infrastructure, is reshaping the demand landscape for live attenuated vaccines across both pediatric and adult populations
- This trend toward global vaccine equity and innovation in delivery systems is fundamentally transforming the accessibility and impact of immunization programs. As a result, leading companies such as GlaxoSmithKline, Sanofi, and Merck are investing heavily in R&D and expanding their global footprint to meet the surging demand
Live Attenuated Vaccines Market Dynamics
Driver
“Growing Need Due to Rising Disease Burden and Public Health Initiatives”
- The increasing global burden of infectious diseases, especially in low- and middle-income countries, combined with expanding public health initiatives, is a major driver for the rising demand for live attenuated vaccines
- For instance, in April 2024, the World Health Organization (WHO) announced the expansion of its Measles & Rubella Initiative across Sub-Saharan Africa, supported by live attenuated vaccine campaigns. Such strategies by key global stakeholders are expected to drive the Live Attenuated Vaccines market growth during the forecast period
- As governments and international health organizations intensify efforts to eradicate vaccine-preventable diseases, live attenuated vaccines offer proven long-lasting immunity with fewer doses, making them cost-effective solutions for large-scale immunization programs
- Furthermore, the ability of live attenuated vaccines to elicit strong cellular and humoral immune responses enhances their effectiveness, particularly in pediatric populations, driving their inclusion in national immunization schedules
- Their utility in both preventive and outbreak-response settings, such as yellow fever or oral polio vaccine campaigns, and the growing focus on global vaccine equity, are key factors propelling their adoption. Increasing public-private partnerships and funding from entities like GAVI and CEPI further contribute to accelerating access and boosting production capacities worldwide
Restraint/Challenge
“Cold Chain Requirements and Risk in Immunocompromised Populations”
- The reliance on strict cold chain logistics for the storage and transportation of live attenuated vaccines presents a significant challenge, especially in remote or resource-limited regions. Maintaining the required temperature range from production to administration can be logistically complex and cost-intensive
- For instance, disruptions in vaccine delivery during the COVID-19 pandemic highlighted the vulnerability of global cold chain infrastructure, impacting the timely availability of temperature-sensitive vaccines
- In addition, live attenuated vaccines, while generally safe for the majority of the population, carry a potential risk when administered to immunocompromised individuals, such as those undergoing chemotherapy or living with HIV/AIDS. This safety concern limits their use in certain patient populations and necessitates careful screening
- Regulatory scrutiny and the need for robust post-marketing surveillance further increase development and deployment timelines. Moreover, limited awareness about vaccine contraindications among healthcare providers in underserved regions can pose risks and hamper public trust
- Overcoming these challenges will require investments in next-generation thermostable vaccine formulations, broader education of healthcare professionals, and expanded infrastructure for temperature-controlled logistics. Continued R&D focused on improving vaccine safety profiles for broader applicability will be vital for long-term market growth.
Live Attenuated Vaccines Market Scope
The market is segmented on the basis of product type, development, indication, mode of administration, by distribution channel and end user.
• By Product Type
On the basis of product type, the live attenuated vaccines market is segmented into bacterial and viral vaccines. The viral segment dominated the market with the largest revenue share of 64.7% in 2024, driven by its widespread application in immunization programs for diseases such as measles, rotavirus, and yellow fever. Viral vaccines are favored for their strong immunogenicity and ability to confer long-lasting protection with fewer doses.
The bacterial segment is expected to witness the fastest CAGR 8.9% from 2025 to 2032, fueled by the increasing prevalence of tuberculosis and rising investments in bacterial vaccine research and development. The resurgence of bacterial infections in developing regions further propels demand.
• By Development
On the basis of development, the live attenuated vaccines market is segmented into tissue culture, embryonated eggs, and live animals. The tissue culture segment held the largest market share of 58.1% in 2024, owing to its scalability, consistency, and reduced risk of contamination. This method is widely used for developing viral vaccines such as measles and rotavirus.
The embryonated eggs segment is projected to grow at the fastest CAGR 9.4% from 2025 to 2032, supported by its continued use in the production of influenza and yellow fever vaccines, particularly in resource-limited settings where infrastructure for cell culture may be lacking.
• By Indication
On the basis of indication, the live attenuated vaccines market is segmented into tuberculosis, measles, rotavirus, yellow fever, oral polio, and others. The measles segment dominated the market in 2024 with a share of 30.3%, supported by widespread global vaccination programs, particularly in children, and strong support from organizations such as WHO and UNICEF.
The rotavirus segment is anticipated to register the fastest CAGR 10.1% from 2025 to 2032, driven by the increasing burden of diarrheal diseases in infants and young children and the expanded rollout of rotavirus vaccines in low-income countries.
• By Mode of Administration
On the basis of mode of administration, the live attenuated vaccines market is segmented into injectable, oral, and others. The injectable segment captured the largest market share of 65.7% in 2024, due to its use in delivering most live attenuated vaccines, including BCG (for tuberculosis), measles, and yellow fever. Injectable vaccines are also widely accepted in clinical settings.
The oral segment is expected to grow at the fastest CAGR 9.8% during the forecast period, owing to its non-invasive nature and ease of administration, particularly in pediatric vaccination programs such as oral polio and rotavirus vaccines.
• By Distribution Channel
On the basis of distribution channel, the live attenuated vaccines market is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. Hospital pharmacies held the largest revenue share in 2024 with share of 51.6% , supported by their critical role in immunization campaigns, access to cold chain infrastructure, and presence within public health institutions.
The online pharmacies segment is projected to witness the fastest growth from 2025 to 2032 with a CAGR of 11.3%, driven by rising e-commerce penetration, growing consumer preference for home delivery, and improved logistics for handling temperature-sensitive products.
• By End User
On the basis of end user, the live attenuated vaccines market is segmented into hospitals, homecare, specialty clinics, and others. The hospitals segment accounted for the largest market share of 57.4 in 2024, as hospitals remain the primary centers for mass immunization and vaccination drives, especially in public health programs.
The specialty clinics segment is forecasted to grow at the fastest CAGR 9.7% during the forecast period, driven by their expanding role in preventive care and their ability to provide focused immunization services with high patient turnover.
Live Attenuated Vaccines Market Regional Analysis
- North America dominates the live attenuated vaccines market with the largest revenue share of 40.01% in 2024, driven by the strong presence of well-established immunization programs, advanced healthcare infrastructure, and high public awareness regarding vaccine-preventable diseases
- The region benefits from widespread government support, frequent vaccine campaigns, and robust surveillance systems, which contribute to the consistent demand for live attenuated vaccines, particularly for measles, mumps, rubella (MMR), and influenza
- In addition, the presence of major market players, extensive R&D funding, and early adoption of next-generation vaccine technologies further solidify North America’s leading position in the market. Increasing pediatric vaccination coverage and travel-related immunization needs also continue to fuel regional growth
U.S. Live Attenuated Vaccines Market Insight
The U.S. live attenuated vaccines market captured the largest revenue share of 79% within North America in 2024, driven by the country’s well-established immunization infrastructure, extensive public health programs, and early adoption of new vaccine technologies. Key federal initiatives such as the Vaccines for Children (VFC) program and strong CDC support ensure widespread usage of live attenuated vaccines, especially for measles, mumps, rubella (MMR), and varicella. Continued R&D efforts by companies like Merck and Sanofi, and regulatory incentives, further support market leadership.
Europe Live Attenuated Vaccines Market Insight
The Europe live attenuated vaccines market is projected to expand at a CAGR of 6.4% from 2025 to 2032, driven by robust government vaccination mandates, increasing awareness of infectious diseases, and the focus on pandemic preparedness. Countries like Germany, France, and Italy have high vaccine coverage rates and well-funded national immunization programs. The European Medicines Agency (EMA)’s strong regulatory framework ensures efficient vaccine rollout. Increasing demand for MMR, rotavirus, and yellow fever vaccines further stimulates growth across the region.
U.K. Live Attenuated Vaccines Market Insight
The U.K. live attenuated vaccines market is anticipated to grow at a CAGR of 6.7% during 2025–2032, fueled by public health campaigns from the NHS, strong government focus on childhood immunization, and rising demand for travel vaccines such as yellow fever. The increasing occurrence of measles outbreaks in certain areas has prompted the government to reinforce uptake of the MMR vaccine, thus boosting market demand.
Germany Live Attenuated Vaccines Market Insight
The Germany live attenuated vaccines market is expected to grow at a CAGR of 6.3% during the forecast period, owing to the country's proactive immunization schedule, state-funded vaccine programs, and rising support for R&D in live vaccine technologies. A high percentage of childhood vaccination coverage and efforts to eliminate diseases like measles and rubella fuel the market.
Asia-Pacific Live Attenuated Vaccines Market Insight
The Asia-Pacific live attenuated vaccines market is poised to grow at the fastest CAGR of 8.9% from 2025 to 2032, accounting for a revenue share of 24.1% in 2024. This growth is driven by large pediatric populations, increasing healthcare expenditure, and government immunization programs across China, India, and Southeast Asia. The market benefits from the WHO’s Expanded Programme on Immunization (EPI), which covers several live attenuated vaccines such as oral polio and measles.
China Live Attenuated Vaccines Market Insight
The China live attenuated vaccines accounted for the largest market share in Asia-Pacific in 2024, with a regional revenue contribution of over 41%, owing to its massive vaccination initiatives, strong public health systems, and expanding biopharmaceutical manufacturing capabilities. High birth rates and proactive government policies supporting childhood immunization programs, including free routine vaccines, drive market demand for live attenuated formulations.
Japan Live Attenuated Vaccines Market Insight
The Japan live attenuated vaccines market is growing steadily at a CAGR of 5.8% during the forecast period, supported by advanced healthcare infrastructure, aging population, and rising awareness of disease prevention. The country’s universal health coverage and increased demand for adult immunization (especially for influenza and shingles) further contribute to sustained growth.
Live Attenuated Vaccines Market Share
The Live Attenuated Vaccines industry is primarily led by well-established companies, including:
- GSK plc. (U.K.)
- Merck & Co., Inc. (U.S.)
- Pfizer Inc. (U.S.)
- Astellas Pharma Inc. (Japan)
- Johnson & Johnson Services, Inc. (U.S.)
- CSL Limited (Australia)
- Emergent BioSolutions, Inc. (U.S.)
- MedImmune, LLC (U.S.)
- Sanofi (France)
- Serum Institute of India Pvt. Ltd. (India)
- Bharat Biotech (India)
- Naobios (France)
Latest Developments in Global Live Attenuated Vaccines Market
- In February 2024, Pfizer Inc. announced the expansion of its live attenuated vaccines production facility in the U.S., aimed at increasing output capacity for vaccines targeting measles and rubella. This strategic move is in response to rising global demand and is expected to enhance Pfizer’s distribution capabilities in developing nations, reinforcing its leadership in the live vaccines segment
- In January 2024, Serum Institute of India Pvt. Ltd. launched an affordable, high-efficacy live attenuated rotavirus vaccine tailored for low- and middle-income countries. The initiative aligns with the company's mission to promote equitable access to immunization and strengthens its presence in the global pediatric vaccine market
- In November 2023, Sanofi entered a public-private partnership with the European Commission to develop next-generation live attenuated vaccines for emerging viral diseases. This collaboration emphasizes innovation and preparedness amid rising concerns over global epidemics, positioning Sanofi as a major innovator in the market.
- In September 2023, Merck & Co., Inc. received expanded FDA approval for its live attenuated MMR (measles, mumps, rubella) vaccine to be used in adults aged 50 and above. The approval opens new demographic segments for the product, supporting market growth in the preventive vaccine domain
- In August 2023, GlaxoSmithKline plc (GSK) launched a new live attenuated varicella vaccine in Latin America, addressing unmet needs in childhood immunization programs. This regional expansion is part of GSK’s broader initiative to increase global immunization coverage through public health collaborations
- In July 2023, MedImmune, LLC, the biologics division of AstraZeneca, initiated Phase III clinical trials for its next-gen live attenuated intranasal influenza vaccine. The new formulation aims to offer broader protection against flu strains with improved mucosal immunity, signaling a major step forward in intranasal vaccine delivery technologies.
- In May 2023, Emergent BioSolutions, Inc. signed a contract with the U.S. Department of Health and Human Services to develop and stockpile live attenuated vaccines for biodefense applications, particularly targeting smallpox and other Category A threatsThis move enhances national preparedness and secures the company’s role in government-led health initiatives
- In March 2023, CSL Limited completed the acquisition of a biotechnology startup specializing in live attenuated vaccine platforms using mRNA-assisted attenuation techniques. The acquisition is aimed at bolstering CSL’s R&D capabilities and diversifying its live vaccine pipeline to address emerging infectious diseases
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.