Global Malaria Vaccines Market
Market Size in USD Billion
CAGR :
% 
 USD
325.00 Billion
USD
498.77 Billion
2024
2032
USD
325.00 Billion
USD
498.77 Billion
2024
2032
| 2025 –2032 | |
| USD 325.00 Billion | |
| USD 498.77 Billion | |
|
|
|
Malaria Vaccine Market Analysis
The Malaria Vaccine market has experienced significant growth due to the increasing demand for effective prevention against malaria, particularly in regions where the disease burden is high. Key factors driving the market include the rising global awareness of malaria's impact, especially in sub-Saharan Africa, and the need for an alternative to traditional methods such as bed nets and antimalarial drugs.
One of the most notable developments is the introduction of RTS,S/AS01, the first malaria vaccine recommended by the World Health Organization (WHO), which has catalyzed further research and development in this field. Pharmaceutical companies are investing heavily in R&D to develop more effective vaccines with broader protection, aiming to overcome limitations such as varying efficacy across age groups and parasite strains.
The market is also influenced by government and NGO initiatives, including global funding and collaboration with public health organizations, which support vaccine distribution and implementation. The rise in private investments and partnerships between governments and pharmaceutical companies has accelerated vaccine development and production.
Despite challenges such as high manufacturing costs and the complexity of vaccine delivery, the growing need for long-term solutions and innovations in vaccine formulations is expected to drive further growth in the Malaria Vaccine market.
Malaria Vaccine Market Size
The global Malaria Vaccine market size was valued at USD 325 million in 2024 and is projected to reach USD 498.77 million by 2032, with a CAGR of 5.50% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Malaria Vaccine Market Trends
“Increased Focus on Multi-Dose and Combination Vaccines”
A key market trend in the Malaria Vaccine market is the increased focus on multi-dose and combination vaccines. With the introduction of RTS,S/AS01, the first malaria vaccine, there has been a growing recognition of the importance of ensuring longer-lasting protection against the malaria parasite. However, due to the challenges of the disease's evolving strains and the complexities of immunity development, there is a shift toward developing vaccines that provide broader protection through multiple doses or by combining malaria vaccines with those for other diseases.
This trend is driven by the need for more comprehensive solutions to combat malaria and reduce the burden of co-infections. Research efforts are being directed towards designing combination vaccines that target multiple pathogens, making it easier for populations to receive protection against multiple diseases in a single treatment. This innovation is expected to improve vaccine adherence and overall effectiveness in high-risk regions.
Report Scope and Malaria Vaccine Market Segmentation
|
Attributes |
Malaria Vaccine Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America |
|
Key Market Players |
Amneal Pharmaceuticals LLC. (U.S.), Ajanta Pharma Ltd. (India), Actiza Pharmaceutical Private Limited (India), AdvaCare Pharma (U.S.), Ipca Laboratories Ltd. (India), Bliss GVS Pharma Ltd. (India), GeoVax (U.S.), Sumaya Biotech (Germany), VLP Therapeutics (U.S.), Osivax (France), Strides Pharma Science Limited (India), Viatris Inc. (U.S.), Cipla (India), Sun Pharmaceutical Industries Ltd. (India), Sanofi (France), Hikma Pharmaceuticals PLC (U.K.), Taj Pharmaceuticals Limited (India), Lupin (India), Novartis AG (Switzerland), Pfizer Inc. (U.S.), Zydus Group (India) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Malaria Vaccine Market Definition
A Malaria vaccine is a biological preparation designed to provide immunity or protection against malaria, a life-threatening disease caused by the Plasmodium parasite, transmitted through the bite of infected Anopheles mosquitoes. The vaccine works by stimulating the immune system to recognize and fight the malaria-causing parasite, either by targeting specific stages of the parasite's life cycle or preventing the parasite from entering the bloodstream.
The goal of malaria vaccines is to reduce the incidence of malaria infections, especially in regions where the disease is most prevalent, such as sub-Saharan Africa. RTS,S/AS01 is currently the most widely recognized malaria vaccine, offering partial protection and being used in select malaria-endemic areas. Ongoing research aims to improve the efficacy and scope of malaria vaccines, with efforts to develop more effective, long-lasting, and accessible solutions to combat the disease globally.
Malaria Vaccine Market Dynamics
Drivers
- Increasing Global Investment in Malaria Eradication Programs
One of the primary drivers of the malaria vaccine market is the growing global investment in malaria eradication efforts. Governments, NGOs, and international organizations such as the WHO and the Global Fund have ramped up funding to combat malaria through research, distribution, and prevention initiatives. A notable instance is the RTS,S/AS01 malaria vaccine, supported by significant investments from entities such as the Bill & Melinda Gates Foundation. This financial backing has accelerated vaccine development, clinical trials, and vaccine rollouts in malaria-endemic regions. As a result, these investments have enhanced both the availability and accessibility of malaria vaccines, fostering market growth and encouraging further innovation. The sustained funding is expected to fuel market expansion, increasing access to vaccines and supporting the development of next-generation vaccines with better efficacy and broader protection.
- Rising Malaria Prevalence and Public Awareness
The increasing prevalence of malaria, especially in sub-Saharan Africa, has heightened the need for effective preventive measures such as vaccination. Malaria continues to be a major cause of morbidity and mortality in many countries, creating urgency for long-term solutions. For instance, in countries such as Ghana, Kenya, and Malawi, the introduction of the RTS,S/AS01 vaccine has been a pivotal moment in malaria control. Public awareness campaigns about the importance of vaccination have gained momentum, leading to greater acceptance and demand for malaria vaccines. This awareness not only promotes vaccine adoption but also stimulates governments and private players to prioritize funding for malaria vaccination programs. As awareness grows and the need for effective prevention increases, the malaria vaccine market continues to expand, encouraging both public and private sector engagement.
Opportunities
- Expansion of Vaccine Coverage in High-Risk Regions
One significant opportunity in the malaria vaccine market is the expansion of vaccine coverage in high-risk regions, particularly in sub-Saharan Africa. Countries with the highest malaria burdens are increasingly focusing on vaccine accessibility to protect vulnerable populations. The rollout of the RTS,S/AS01 malaria vaccine in pilot programs in Ghana, Malawi, and Kenya has demonstrated positive results, with a marked reduction in malaria cases. These initiatives have sparked interest in further expanding vaccination programs across other malaria-endemic countries. In addition, the WHO’s endorsement of RTS,S/AS01 as a valuable tool in malaria prevention opens doors for similar vaccines to be introduced, resulting in increased market demand. As more countries adopt comprehensive vaccination strategies, the market for malaria vaccines is expected to see substantial growth, making this expansion a key opportunity for both vaccine manufacturers and global health organizations.
- Innovation in Malaria Vaccine Development
Another promising opportunity lies in the continuous innovation in malaria vaccine development. Researchers are focusing on creating more effective vaccines that offer longer-lasting immunity and broader protection against various malaria strains. The development of new vaccines such as the R21/Matrix-M vaccine, which has shown higher efficacy in clinical trials, could revolutionize malaria prevention. The successful progress of these innovative vaccine candidates holds the potential to address the limitations of existing vaccines, such as partial protection and the need for multiple doses. As innovation continues, both public and private sector investments are likely to increase, which will drive competition and growth in the market. With the success of new vaccines, the malaria vaccine market will experience accelerated growth, providing more comprehensive protection against malaria and reaching a wider global population.
Restraints/Challenges
- Limited Vaccine Storage and Distribution Infrastructure
A significant restraint in the malaria vaccine market is the limited infrastructure for vaccine storage and distribution, particularly in remote or rural areas of malaria-endemic countries. Malaria vaccines, such as RTS,S/AS01, require stringent cold-chain storage to maintain their efficacy, which can be challenging in regions with inadequate infrastructure. In many low-income countries, especially in sub-Saharan Africa, power shortages, lack of refrigeration facilities, and logistical difficulties make it difficult to transport and store vaccines properly. For instance, while organizations such as GAVI have been working to improve vaccine distribution systems, the infrastructure gap remains a barrier. This restraint hampers the widespread delivery of vaccines, slowing down market growth. Until investments are made in strengthening healthcare systems, particularly in rural regions, the distribution of malaria vaccines will face considerable challenges, limiting their overall market potential.
- Variability in Efficacy Across Different Strains of Malaria
A major challenge facing the malaria vaccine market is the variability in vaccine efficacy across different Plasmodium parasite strains. Malaria is caused by several Plasmodium species, with Plasmodium falciparum being the deadliest. However, vaccines such as RTS,S/AS01 have shown varying degrees of effectiveness depending on the parasite strain and age group. For instance, RTS,S/AS01 has demonstrated limited protection in children under five, the most vulnerable group. This challenge means that a single vaccine may not offer universal protection across all malaria-endemic areas, complicating vaccine deployment and uptake. Efforts to develop more universally effective vaccines are underway, but until a broadly effective vaccine is available, this challenge will persist. This issue slows market growth.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Malaria Vaccine Market Scope
The market is segmented on the basis of agent, vaccine type, route of administration, end-users, and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Agent
- Plasmodium Falciparum
- Plasmodium Vivax
- Anopheles Species
Vaccine Type
- Pre-Erythrocytic
- Erythrocytic
- Multi-antigen
- Others
Route of Administration
- Intramuscular
- Subcutaneous
- Intradermal
- Others
End-Users
- Hospitals
- Specialty Clinics
- Homecare
- Others
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
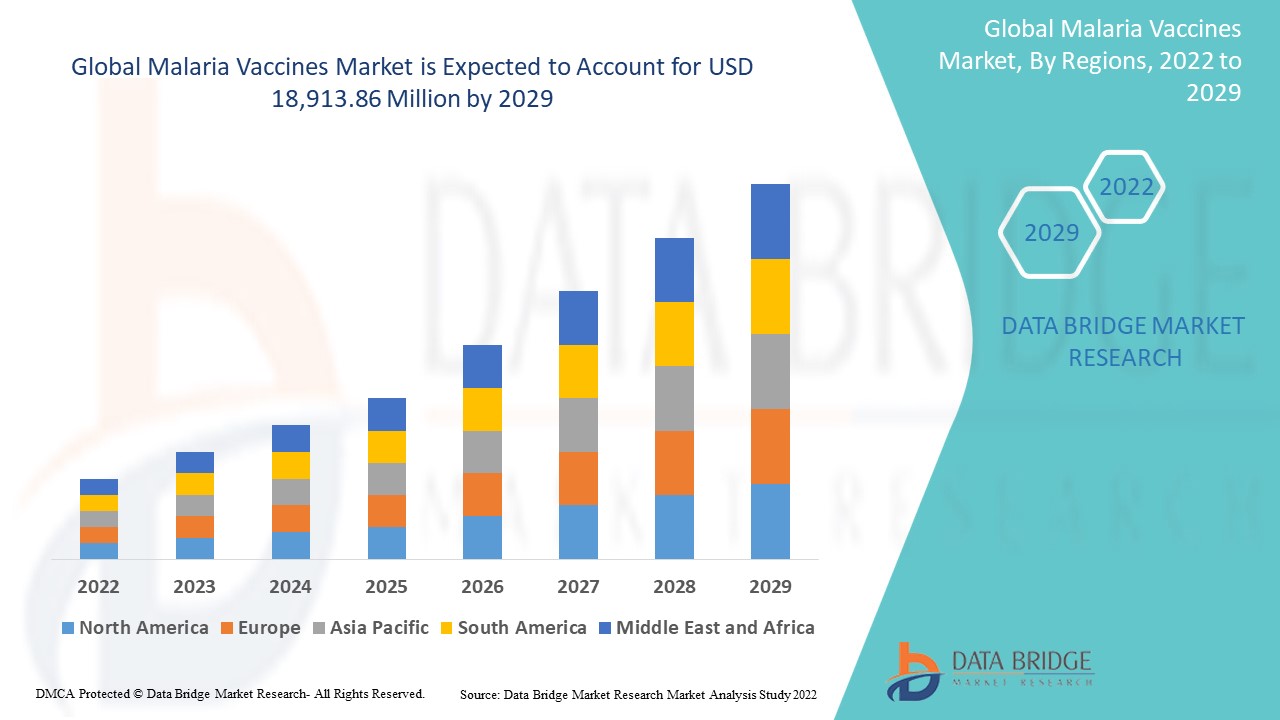
Malaria Vaccine Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, agent, vaccine type, route of administration, end-users, and distribution channel as referenced above.
The countries covered in the market report are U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America.
North America dominates the malaria vaccines market because of the presence of key manufacturers of the product and well-established healthcare infrastructure in this region. In addition, high research and development expenditure and presence of skilled professionals will further propel the market’s growth rate in this region.
Asia-Pacific is expected to exhibit the highest growth rate in the Malaria Vaccine Market. This is primarily driven by the rising investment in healthcare infrastructure, increasing malaria prevalence in certain areas, and the ongoing efforts to expand vaccination programs. Countries such as India, Indonesia, and parts of Southeast Asia face significant malaria burdens, and with growing government support for malaria eradication programs, the demand for vaccines is expected to rise.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Malaria Vaccine Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Malaria Vaccine Market Leaders Operating in the Market Are:
- Amneal Pharmaceuticals LLC. (U.S.)
- Ajanta Pharma Ltd. (India)
- Actiza Pharmaceutical Private Limited (India)
- AdvaCare Pharma (U.S.)
- Ipca Laboratories Ltd. (India)
- Bliss GVS Pharma Ltd. (India)
- GeoVax (U.S.)
- Sumaya Biotech (Germany)
- VLP Therapeutics (U.S.)
- Osivax (France)
- Strides Pharma Science Limited (India)
- Viatris Inc. (U.S.)
- Cipla (India)
- Sun Pharmaceutical Industries Ltd. (India)
- Sanofi (France)
- Hikma Pharmaceuticals PLC (U.K.)
- Taj Pharmaceuticals Limited (India)
- Lupin (India)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- Zydus Group (India)
Latest Developments in Malaria Vaccine Market
- In July 2024, the Serum Institute of India (SII) and Oxford University introduced the "high efficacy" malaria vaccine, R21/Matrix-M, in Côte d'Ivoire. Approved by the WHO, this vaccine is not only effective and affordable but also capable of being mass-produced rapidly. SII plans to manufacture 100 million doses annually, with each dose priced at under USD 4
- In May 2024, a team of scientists from Jawaharlal Nehru University (JNU) identified a promising vaccine candidate that could lead to more effective prevention and treatment strategies for malaria. Their research, published in the iScience journal by Cell Press, suggests targeting the parasite's Prohibitin protein as a novel approach for vaccine development
- In May 2024, the Serum Institute of India commenced exports of the 'R21/Matrix-M' malaria vaccine to Africa. Developed in partnership with the University of Oxford and Novavax’s Matrix-M adjuvant, the vaccine marks a significant step in malaria prevention. On Monday, May 20, Adar Poonawalla and the U.S. Ambassador to India, Eric Garcetti, officially launched the first shipment of R21/Matrix-M malaria vaccine doses
- In October 2023, the World Health Organization (WHO) recommended the R21/Matrix-M vaccine for malaria prevention in children. This recommendation came after guidance from the WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) and the Malaria Policy Advisory Group (MPAG), and was officially endorsed by the WHO Director-General following their regular biannual meeting held from September 25-29
- In January 2021, vaccine manufacturer Bharat Biotech signed a product transfer agreement with pharmaceutical giant GSK and the global non-profit organization PATH to produce the world’s only malaria vaccine, RTS,S/AS01E1, developed by GSK. Under the agreement, Bharat Biotech will take over the manufacturing of the RTS,S antigen component and the vaccine itself
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.