Global Maternal Blood Test Market
Market Size in USD Billion
CAGR :
% 
 USD
3.81 Billion
USD
10.12 Billion
2022
2030
USD
3.81 Billion
USD
10.12 Billion
2022
2030
| 2023 –2030 | |
| USD 3.81 Billion | |
| USD 10.12 Billion | |
|
|
|
|
Maternal Blood Test Market Analysis and Size
One of the major factors driving the growth of the maternal blood test market is the continuous improvement in the reimbursement scenario for NIPT around the world. An increase in the incidence of chromosomal abnormalities, combined with increasing product usage in new applications, drive market growth. The growing preference for non-invasive techniques over invasive methods, as well as an increase in programmes aimed at raising NIPT awareness, all have an impact on the market.
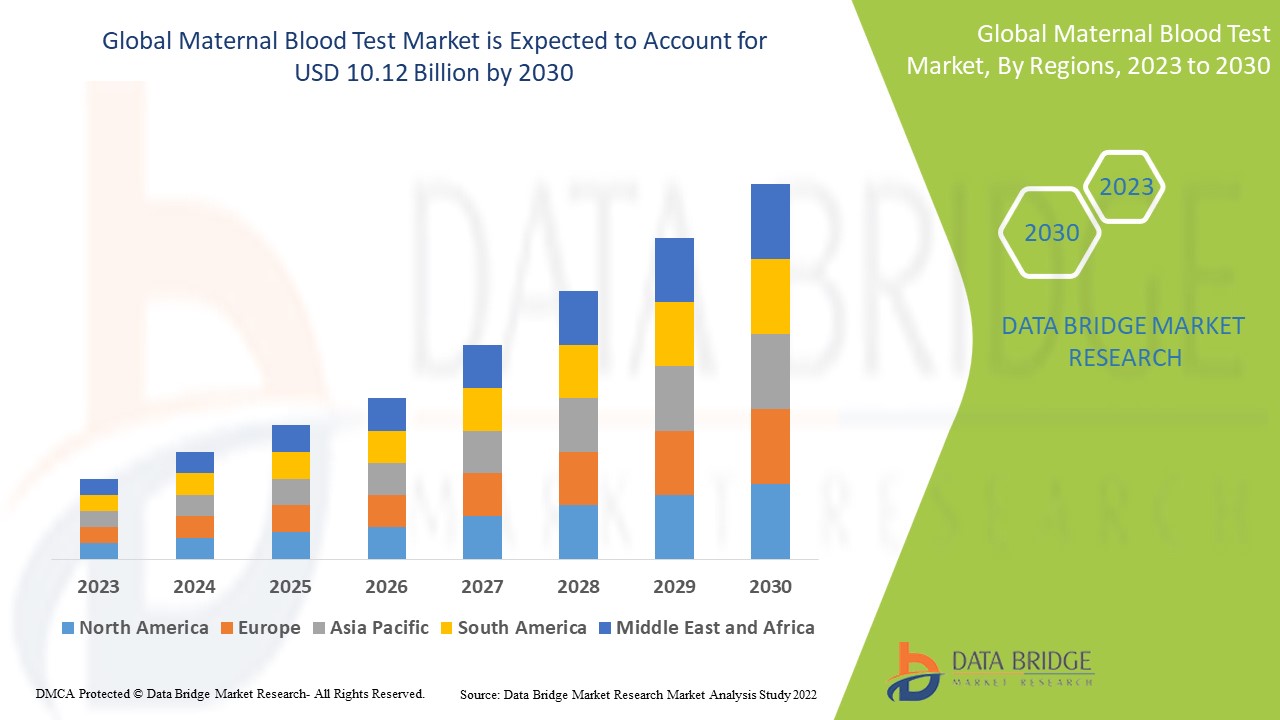
Data Bridge Market Research analyses that the maternal blood test market which was USD 3.807 billion in 2022, is expected to reach USD 10.12 billion by 2030, at a CAGR of 13.00% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Maternal Blood Test Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Billion, Volumes in Units, Pricing in USD |
|
Segments Covered |
Type (Combined First Trimester Screening, Second Trimester Maternal Serum Screening), Tested Conditions (Edwards Syndrome, Patau Syndrome, Down syndrome, Neural Tube Defects), End- User (Diagnostic Laboratories, Hospitals) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
CENTOGENE N.V. (Germany), SEQUENOM (U.S.), Natera, Inc. (U.S.), LifeLabs Genetics (Canada), Sema4 OpCo, Inc. (U.S.), Invitae Corporation (U.S.), Illumina, Inc. (U.S.), Thermo Fisher Scientific Inc. (U.S.), General Electric (U.S.), PerkinElmer Inc. (U.S.), Agilent Technologies, Inc. (U.S.), BGI (China), F. Hoffmann-La Roche Ltd (Switzerland), YOURGENE HEALTH (U.K.), Eurofins Scientific (Luxembourg), EKF Diagnostics (Germany) |
|
Market Opportunities |
|
Market Definition
A maternal blood test is a type of prenatal screening test that determines the likelihood that a chromosome disorder will impact a pregnancy or certain behavioural issues will impact a pregnancy. The primary goal of the test is to ensure that the baby does not have any chromosomal abnormalities such as Down syndrome. These tests are known to be capable of providing more information about pregnancy health.
Global Maternal Blood Test Market Dynamics
Drivers
- Rising use of maternal blood tests
Maternal blood tests can detect and diagnose chromosomal rearrangements, X-linked diseases, and aid in the reduction of spontaneous abortions, increased implantation rates, the prevention of trisomic offspring, and the avoidance of the risk of transmitting single gene disorders. However, PGS and PGD results are not 100% accurate, and if necessary, diagnostic tests such as amniocentesis and CVS must be performed after pregnancy to confirm the positive PGS/PGD results. In the United States alone, more than 63,000 babies were born through IVF in 2013, an increase of 2,000 births from 2012. These are the certain test that help the market to grow.
Opportunities
- Introduction of NIPTs
The introduction of NIPTs using cell-free foetal DNA (cffDNA) in 2011 was the most significant advancement in the field of prenatal screening. Obstetricians and patients who are put off by ultrasound screening's 10% to 15% false positive rate and maternal serum test's 5% false positive rate are eagerly embracing NIPTs that claim to have only a 5% false positive rate. In just four years, molecular genetics companies have successfully developed eight different types of kits for detecting genetic abnormalities in foetus. These products have gradually marginalized maternal serum tests, and maternal serum tests are expected to become obsolete within the next decade.
Restraints/Challenges
- Lack of skilled professionals
Lack of skilled and trained professionals, strict regulatory guidelines and ethical stumbling blocks limits the market's growth during the forecast period.
This maternal blood test market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the maternal blood test market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Global Maternal Blood Test Market Scope
The maternal blood test market is segmented on the basis of type, tested condition and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Combined First Trimester Screening
- Second Trimester Maternal Serum Screening
Tested Conditions
- Edwards Syndrome
- Patau Syndrome
- Down syndrome
- Neural Tube Defects
End-User
- Diagnostic Laboratories
- Hospitals
Maternal Blood Test Market Regional Analysis/Insights
The maternal blood test market is analyzed and market size insights and trends are provided by country, type, tested condition and end-user as referenced above.
The countries covered in the maternal blood test market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the maternal blood test market because of the high adoption of advanced technologies within the region.
Asia-Pacific is expected to grow at the highest growth rate in the forecast period of 2023 to 2030 owing to the developing healthcare infrastructure, and increasing awareness programs and conferences in the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed base and New Technology Penetration
The maternal blood test market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for maternal blood test market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the maternal blood test market. The data is available for historic period 2011-2021.
Competitive Landscape and Maternal Blood Test Market Share Analysis
The maternal blood test market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to maternal blood test market.
Some of the major players operating in the maternal blood test market are:
- CENTOGENE N.V. (Germany)
- SEQUENOM (U.S.)
- Natera, Inc. (U.S.)
- LifeLabs Genetics (Canada)
- Sema4 OpCo, Inc. (U.S.)
- Invitae Corporation (U.S.)
- Illumina, Inc. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- General Electric (U.S.)
- PerkinElmer Inc. (U.S.)
- Agilent Technologies, Inc. (U.S.)
- BGI (China)
- F. Hoffmann-La Roche Ltd (Switzerland)
- YOURGENE HEALTH (U.K.)
- Eurofins Scientific (Luxembourg)
- EKF Diagnostics (Germany)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL MATERNAL BLOOD TEST MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL MATERNAL BLOOD TEST MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES VOLUME DATA
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL MATERNAL BLOOD TEST MARKET : RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNONLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 MERGERS AND ACQUISITIONS
10.8 FUTURE OUTLOOK
11 REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR GLOBAL MATERNAL BLOOD TEST MARKET
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
12 REIMBURSEMENT FRAMEWORK
13 OPPUTUNITY MAP ANALYSIS
14 VALUE CHAIN ANALYSIS
15 HEALTHCARE ECONOMY
15.1 HEALTHCARE EXPENDITURE
15.2 CAPITAL EXPENDITURE
15.3 CAPEX TRENDS
15.4 CAPEX ALLOCATION
15.5 FUNDING SOURCES
15.6 INDUSTRY BENCHMARKS
15.7 GDP RATION IN OVERALL GDP
15.8 HEALTHCARE SYSTEM STRUCTURE
15.9 GOVERNMENT POLICIES
15.1 ECONOMIC DEVELOPMENT
16 GLOBAL MATERNAL BLOOD TEST MARKET, BY PRODUCT AND SERVICES
16.1 OVERVIEW
16.2 PRODUCT
16.2.1 TEST KITS
16.2.1.1. NIPT KITS
16.2.1.1.1. SINGLE-GENE DISORDER KITS
16.2.1.1.2. MULTI-GENE PANEL KITS
16.2.1.1.3. CFDNA-BASED KITS
16.2.1.2. CARRIER SCREENING KITS
16.2.1.2.1. ETHNICITY-SPECIFIC CARRIER SCREENING KITS
16.2.1.2.2. EXPANDED CARRIER SCREENING KITS
16.2.1.3. BIOCHEMICAL SCREENING KITS
16.2.1.3.1. TRIPLE MARKER KITS
16.2.1.3.2. QUADRUPLE MARKER KITS
16.2.1.4. COMBINED SCREENING KITS
16.2.1.4.1. FIRST TRIMESTER COMBINED KITS
16.2.1.4.2. SEQUENTIAL SCREENING KITS
16.2.1.5. RAPID TEST KITS
16.2.2 INSTRUMENTS
16.2.2.1. DIAGNOSTIC DEVICES
16.2.2.1.1. SEQUENCING MACHINES (NGS-BASED AND SANGER SEQUENCING)
16.2.2.1.2. PCR DEVICES (REAL-TIME AND DIGITAL PCR)
16.2.2.1.3. FLUORESCENCE IN SITU HYBRIDIZATION (FISH) SYSTEMS
16.2.2.2. AUTOMATION PLATFORMS
16.2.2.2.1. ROBOTIC LIQUID HANDLERS FOR HIGH-THROUGHPUT TESTING
16.2.2.2.2. SAMPLE PREPARATION INSTRUMENTS
16.2.2.2.3. OTHERS
16.2.2.3. IMMUNOASSAY SYSTEMS
16.2.2.3.1. ELISA READERS
16.2.2.3.2. CHEMILUMINESCENCE ANALYZERS
16.2.3 CONSUMABLES
16.2.3.1. COLLECTION MATERIALS
16.2.3.1.1. SPECIALIZED BLOOD COLLECTION TUBES FOR CFDNA STABILITY
16.2.3.1.2. SERUM/PLASMA COLLECTION SYSTEMS
16.2.3.2. REAGENTS AND BUFFERS
16.2.3.2.1. PCR REAGENTS
16.2.3.2.2. ANTIBODY REAGENTS FOR BIOCHEMICAL ASSAYS
16.2.3.3. SAMPLE PREPARATION CONSUMABLES
16.2.3.3.1. DNA/RNA EXTRACTION KITS
16.2.3.3.2. MICROARRAY PREPARATION KITS
16.2.3.4. SINGLE-USE ITEMS
16.2.3.4.1. DISPOSABLE PIPETTES
16.2.3.4.2. SPECIALIZED REACTION PLATES
16.3 SERVICES AND SOFTWARE
17 GLOBAL MATERNAL BLOOD TEST MARKET, BY TEST TYPE
17.1 OVERVIEW
17.2 NON-INVASIVE PRENATAL TESTING (NIPT)
17.2.1 CELL-FREE DNA (CFDNA) TESTING
17.2.2 CHROMOSOMAL ANEUPLOIDY SCREENING
17.2.3 MICRODELETIONS AND MICRODUPLICATIONS TESTING
17.3 CARRIER SCREENING TESTS
17.3.1 SINGLE-GENE DISORDERS
17.3.2 MULTI-GENE PANELS
17.4 BIOCHEMICAL SCREENING
17.4.1 TRIPLE MARKER TESTS
17.4.2 QUADRUPLE MARKER TESTS
17.5 COMBINED FIRST TRIMESTER SCREENING
17.6 MATERNAL SERUM SCREENING
17.7 OTHERS
18 GLOBAL MATERNAL BLOOD TEST MARKET, BY TECHNOLOGY
18.1 OVERVIEW
18.2 POLYMERASE CHAIN REACTION (PCR)
18.3 NEXT-GENERATION SEQUENCING (NGS)
18.4 MICROARRAY TECHNOLOGY
18.5 IMMUNOASSAYS
18.6 FLUORESCENCE IN SITU HYBRIDIZATION (FISH)
18.7 OTHERS
19 GLOBAL MATERNAL BLOOD TEST MARKET, BY APPLICATION
19.1 OVERVIEW
19.2 FETAL ANEUPLOIDY DETECTION
19.2.1 DOWN SYNDROME (TRISOMY 21)
19.2.2 EDWARDS SYNDROME (TRISOMY 18)
19.2.3 PATAU SYNDROME (TRISOMY 13)
19.3 GENDER DETERMINATION
19.4 SCREENING FOR MONOGENIC DISORDERS
19.5 DETECTION OF MICRODELETIONS/MICRODUPLICATIONS
19.6 RHD AND BLOOD GROUP TYPING
19.7 SCREENING FOR PRE-ECLAMPSIA AND GESTATIONAL DIABETES
19.8 OTHERS
20 GLOBAL MATERNAL BLOOD TEST MARKET, BY SCREENING TYPE
20.1 OVERVIEW
20.2 ROUTINE SCREENING
20.3 HIGH-RISK PREGNANCY SCREENING
20.3.1 ADVANCED MATERNAL AGE
20.3.2 FAMILY HISTORY OF GENETIC DISORDERS
20.3.3 RECURRENT PREGNANCY LOSS
21 GLOBAL MATERNAL BLOOD TEST MARKET, BY TRIMESTER
21.1 OVERVIEW
21.2 FIRST TRIMESTER
21.3 SECOND TRIMESTER
21.4 THIRD TRIMESTER
22 GLOBAL MATERNAL BLOOD TEST MARKET, BY END USER
22.1 OVERVIEW
22.2 HOSPITALS
22.3 DIAGNOSTIC LABORATORIES
22.4 MATERNITY CLINICS
22.5 HOME CARE SETTINGS
22.6 RESEARCH & ACADEMIC INSTITUTIONS
22.7 OTHERS
23 GLOBAL MATERNAL BLOOD TEST MARKET, BY DISTRIBUTION CHANNEL
23.1 OVERVIEW
23.2 DIRECT TENDERS
23.3 RETAIL SALES
23.3.1 ONLINE SALES
23.3.2 OFFLINES SALES
23.4 OTHERS
24 GLOBAL MATERNAL BLOOD TEST MARKET , BY GEOGRAPHY
GLOBAL MATERNAL BLOOD TEST MARKET (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
24.1 ASIA-PACIFIC
24.1.1 JAPAN
24.1.2 CHINA
24.1.3 SOUTH KOREA
24.1.4 INDIA
24.1.5 AUSTRALIA
24.1.6 SINGAPORE
24.1.7 THAILAND
24.1.8 MALAYSIA
24.1.9 INDONESIA
24.1.10 PHILIPPINES
24.1.11 REST OF ASIA-PACIFIC
24.2 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
25 GLOBAL MATERNAL BLOOD TEST MARKET , COMPANY LANDSCAPE
25.1 COMPANY SHARE ANALYSIS: GLOBAL
25.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
25.3 COMPANY SHARE ANALYSIS: EUROPE
25.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
25.5 MERGERS & ACQUISITIONS
25.6 NEW PRODUCT DEVELOPMENT & APPROVALS
25.7 EXPANSIONS
25.8 REGULATORY CHANGES
25.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
26 GLOBAL MATERNAL BLOOD TEST MARKET , SWOT AND DBMR ANALYSIS
27 GLOBAL MATERNAL BLOOD TEST MARKET , COMPANY PROFILE
27.1 BIO-RAD LABORATORIES, INC.
27.1.1 COMPANY OVERVIEW
27.1.2 REVENUE ANALYSIS
27.1.3 GEOGRAPHIC PRESENCE
27.1.4 PRODUCT PORTFOLIO
27.1.5 RECENT DEVELOPMENTS
27.2 ABBOTT
27.2.1 COMPANY OVERVIEW
27.2.2 REVENUE ANALYSIS
27.2.3 GEOGRAPHIC PRESENCE
27.2.4 PRODUCT PORTFOLIO
27.2.5 RECENT DEVELOPMENTS
27.3 SIEMENS HEALTHINEERS AG
27.3.1 COMPANY OVERVIEW
27.3.2 REVENUE ANALYSIS
27.3.3 GEOGRAPHIC PRESENCE
27.3.4 PRODUCT PORTFOLIO
27.3.5 RECENT DEVELOPMENTS
27.4 DANAHER
27.4.1 COMPANY OVERVIEW
27.4.2 REVENUE ANALYSIS
27.4.3 GEOGRAPHIC PRESENCE
27.4.4 PRODUCT PORTFOLIO
27.4.5 RECENT DEVELOPMENTS
27.5 DIASORIN S.P.A.
27.5.1 COMPANY OVERVIEW
27.5.2 REVENUE ANALYSIS
27.5.3 GEOGRAPHIC PRESENCE
27.5.4 PRODUCT PORTFOLIO
27.5.5 RECENT DEVELOPMENTS
27.6 ALPCO
27.6.1 COMPANY OVERVIEW
27.6.2 REVENUE ANALYSIS
27.6.3 GEOGRAPHIC PRESENCE
27.6.4 PRODUCT PORTFOLIO
27.6.5 RECENT DEVELOPMENTS
27.7 BIOASSAY SYSTEMS
27.7.1 COMPANY OVERVIEW
27.7.2 REVENUE ANALYSIS
27.7.3 GEOGRAPHIC PRESENCE
27.7.4 PRODUCT PORTFOLIO
27.7.5 RECENT DEVELOPMENTS
27.8 DIAGNOSTIC AUTOMATION/CORTEZ DIAGNOSTICS INC.
27.8.1 COMPANY OVERVIEW
27.8.2 REVENUE ANALYSIS
27.8.3 GEOGRAPHIC PRESENCE
27.8.4 PRODUCT PORTFOLIO
27.8.5 RECENT DEVELOPMENTS
27.9 DIAZYME LABORATORIES, INC
27.9.1 COMPANY OVERVIEW
27.9.2 REVENUE ANALYSIS
27.9.3 GEOGRAPHIC PRESENCE
27.9.4 PRODUCT PORTFOLIO
27.9.5 RECENT DEVELOPMENTS
27.1 ABNOVA CORPORATION
27.10.1 COMPANY OVERVIEW
27.10.2 REVENUE ANALYSIS
27.10.3 GEOGRAPHIC PRESENCE
27.10.4 PRODUCT PORTFOLIO
27.10.5 RECENT DEVELOPMENTS
27.11 DRG INSTRUMENTS GMBH
27.11.1 COMPANY OVERVIEW
27.11.2 REVENUE ANALYSIS
27.11.3 GEOGRAPHIC PRESENCE
27.11.4 PRODUCT PORTFOLIO
27.11.5 RECENT DEVELOPMENTS
27.12 EKF DIAGNOSTICS HOLDINGS PLC
27.12.1 COMPANY OVERVIEW
27.12.2 REVENUE ANALYSIS
27.12.3 GEOGRAPHIC PRESENCE
27.12.4 PRODUCT PORTFOLIO
27.12.5 RECENT DEVELOPMENTS
27.13 ETHOS BIOSCIENCES, INC.
27.13.1 COMPANY OVERVIEW
27.13.2 REVENUE ANALYSIS
27.13.3 GEOGRAPHIC PRESENCE
27.13.4 PRODUCT PORTFOLIO
27.13.5 RECENT DEVELOPMENTS
27.14 MEDIPAN GMBH
27.14.1 COMPANY OVERVIEW
27.14.2 REVENUE ANALYSIS
27.14.3 GEOGRAPHIC PRESENCE
27.14.4 PRODUCT PORTFOLIO
27.14.5 RECENT DEVELOPMENTS
27.15 MERCK KGAA
27.15.1 COMPANY OVERVIEW
27.15.2 REVENUE ANALYSIS
27.15.3 GEOGRAPHIC PRESENCE
27.15.4 PRODUCT PORTFOLIO
27.15.5 RECENT DEVELOPMENTS
27.16 MONOBIND INC.
27.16.1 COMPANY OVERVIEW
27.16.2 REVENUE ANALYSIS
27.16.3 GEOGRAPHIC PRESENCE
27.16.4 PRODUCT PORTFOLIO
27.16.5 RECENT DEVELOPMENTS
27.17 ORGENTEC DIAGNOSTIKA GMBH
27.17.1 COMPANY OVERVIEW
27.17.2 REVENUE ANALYSIS
27.17.3 GEOGRAPHIC PRESENCE
27.17.4 PRODUCT PORTFOLIO
27.17.5 RECENT DEVELOPMENTS
27.18 ORTHO CLINICAL DIAGNOSTICS
27.18.1 COMPANY OVERVIEW
27.18.2 REVENUE ANALYSIS
27.18.3 GEOGRAPHIC PRESENCE
27.18.4 PRODUCT PORTFOLIO
27.18.5 RECENT DEVELOPMENTS
27.19 PTS DIAGNOSTICS (A SUBSIDIARY OF SANNUO BIOSENSOR CO., LTD.)
27.19.1 COMPANY OVERVIEW
27.19.2 REVENUE ANALYSIS
27.19.3 GEOGRAPHIC PRESENCE
27.19.4 PRODUCT PORTFOLIO
27.19.5 RECENT DEVELOPMENTS
27.2 TOSOH CORPORATION
27.20.1 COMPANY OVERVIEW
27.20.2 REVENUE ANALYSIS
27.20.3 GEOGRAPHIC PRESENCE
27.20.4 PRODUCT PORTFOLIO
27.20.5 RECENT DEVELOPMENTS
27.21 F. HOFFMANN-LA ROCHE AG
27.21.1 COMPANY OVERVIEW
27.21.2 REVENUE ANALYSIS
27.21.3 GEOGRAPHIC PRESENCE
27.21.4 PRODUCT PORTFOLIO
27.21.5 RECENT DEVELOPMENTS
27.22 NOVO NORDISK A/S
27.22.1 COMPANY OVERVIEW
27.22.2 REVENUE ANALYSIS
27.22.3 GEOGRAPHIC PRESENCE
27.22.4 PRODUCT PORTFOLIO
27.22.5 RECENT DEVELOPMENTS
27.23 ARKRAY INC
27.23.1 COMPANY OVERVIEW
27.23.2 REVENUE ANALYSIS
27.23.3 GEOGRAPHIC PRESENCE
27.23.4 PRODUCT PORTFOLIO
27.23.5 RECENT DEVELOPMENTS
27.24 ASCENSIA DIABETES CARE
27.24.1 COMPANY OVERVIEW
27.24.2 REVENUE ANALYSIS
27.24.3 GEOGRAPHIC PRESENCE
27.24.4 PRODUCT PORTFOLIO
27.24.5 RECENT DEVELOPMENTS
27.25 LIFESCAN IP HOLDING, LLC
27.25.1 COMPANY OVERVIEW
27.25.2 REVENUE ANALYSIS
27.25.3 GEOGRAPHIC PRESENCE
27.25.4 PRODUCT PORTFOLIO
27.25.5 RECENT DEVELOPMENTS
27.26 DEXCOM, INC.
27.26.1 COMPANY OVERVIEW
27.26.2 REVENUE ANALYSIS
27.26.3 GEOGRAPHIC PRESENCE
27.26.4 PRODUCT PORTFOLIO
27.26.5 RECENT DEVELOPMENTS
27.27 MEDTRONIC
27.27.1 COMPANY OVERVIEW
27.27.2 REVENUE ANALYSIS
27.27.3 GEOGRAPHIC PRESENCE
27.27.4 PRODUCT PORTFOLIO
27.27.5 RECENT DEVELOPMENTS
27.28 B. BRAUN MELSUNGEN AG
27.28.1 COMPANY OVERVIEW
27.28.2 REVENUE ANALYSIS
27.28.3 GEOGRAPHIC PRESENCE
27.28.4 PRODUCT PORTFOLIO
27.28.5 RECENT DEVELOPMENTS
27.29 TERUMO CORPORATION
27.29.1 COMPANY OVERVIEW
27.29.2 REVENUE ANALYSIS
27.29.3 GEOGRAPHIC PRESENCE
27.29.4 PRODUCT PORTFOLIO
27.29.5 RECENT DEVELOPMENTS
27.3 ACON LABORATORIES, INC.
27.30.1 COMPANY OVERVIEW
27.30.2 REVENUE ANALYSIS
27.30.3 GEOGRAPHIC PRESENCE
27.30.4 PRODUCT PORTFOLIO
27.30.5 RECENT DEVELOPMENTS
28 CONCLUSION
29 QUESTIONNAIRE
30 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.













