Global Rna Interference Rnai Nucleic Acid Therapeutics Market
Market Size in USD Billion
CAGR :
% 
 USD
2.22 Billion
USD
3.86 Billion
2024
2032
USD
2.22 Billion
USD
3.86 Billion
2024
2032
| 2025 –2032 | |
| USD 2.22 Billion | |
| USD 3.86 Billion | |
|
|
|
|
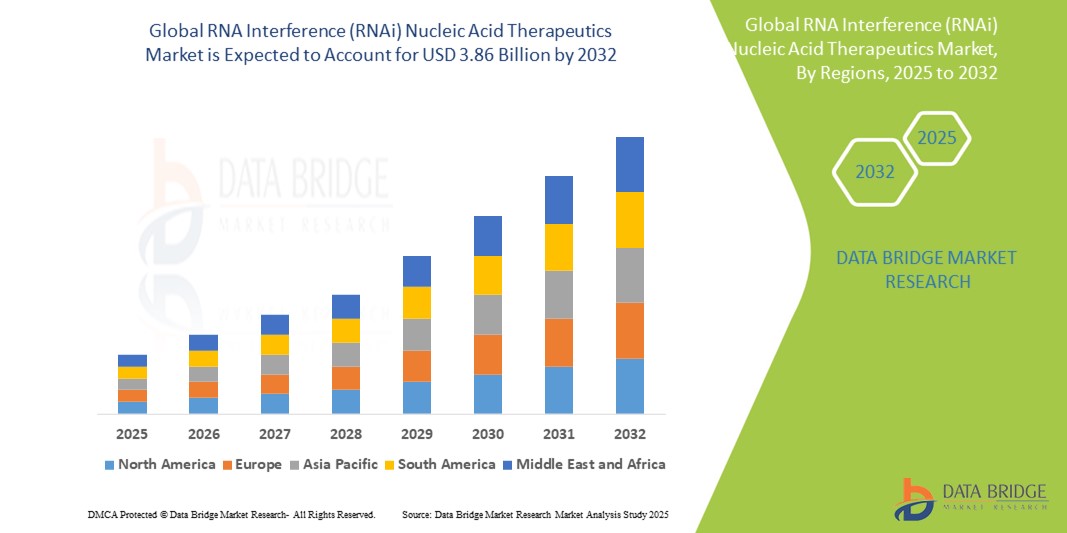
RNA Interference (RNAi) Nucleic Acid Therapeutics Market Size
- The global RNA Interference (RNAi) nucleic acid therapeutics market size was valued at USD 2.22 billion in 2024 and is expected to reach USD 3.86 billion by 2032, at a CAGR of 7.13% during the forecast period
- The market growth is largely fueled by the growing adoption and technological progress within RNA-based drug discovery and gene-silencing platforms, leading to increased development of targeted therapies in both rare and chronic disease segments. RNA interference (RNAi) technology enables precise silencing of specific genes, which is revolutionizing treatment strategies in oncology, metabolic disorders, and genetic diseases
- Furthermore, rising demand for safe, efficient, and personalized therapeutic solutions is establishing RNAi-based drugs as a transformative class of therapeutics. These converging factors are accelerating the uptake of RNA Interference (RNAi) nucleic acid therapeutics solutions, thereby significantly boosting the industry's growth. The increasing number of clinical trials, successful FDA approvals (such as Patisiran and Givosiran), and partnerships between biotech firms and large pharmaceutical companies further underscore the rapid momentum in this market
RNA Interference (RNAi) Nucleic Acid Therapeutics Market Analysis
- RNAi therapeutics, offering targeted gene-silencing mechanisms, are increasingly vital components of modern precision medicine, particularly in treating genetic disorders, cancers, and rare diseases. Their ability to specifically inhibit the expression of disease-causing genes is revolutionizing treatment approaches across both clinical and research settings.
- The escalating demand for RNA Interference (RNAi) nucleic acid therapeutics is primarily fueled by the growing prevalence of chronic and genetic diseases, advancements in oligonucleotide delivery systems, and strong pipeline developments by leading pharmaceutical companies. In Addition, favorable regulatory support and rising R&D investment are contributing to accelerating the commercialization of RNAi-based therapies
- North America dominated the RNA Interference (RNAi) Nucleic Acid Therapeutics market with the largest revenue share of 45.7% in 2024, driven by robust healthcare infrastructure, early adoption of advanced therapeutics, high healthcare expenditure, and strong presence of key industry players such as Alnylam Pharmaceuticals and Arrowhead Pharmaceuticals
- Asia-Pacific is expected to be the fastest-growing region in the RNA Interference (RNAi) Nucleic Acid Therapeutics market during the forecast period, with a projected CAGR of 17.4%, attributed to improving healthcare systems, growing investments in biotechnology, and increasing collaborations between regional pharmaceutical firms and global RNAi drug developers
- The Small Interfering RNA (siRNA) segment dominated the RNA Interference (RNAi) Nucleic Acid Therapeutics market with a market share of 61.3% in 2024, owing to its proven clinical efficacy, well-established delivery systems, and higher number of FDA-approved therapies. siRNA-based drugs such as Onpattro and Givlaari have paved the way for expanding RNAi drug pipelines globally
Report Scope and RNA Interference (RNAi) Nucleic Acid Therapeutics Market Segmentation
|
Attributes |
RNA Interference (RNAi) Nucleic Acid Therapeutics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
RNA Interference (RNAi) Nucleic Acid Therapeutics Market Trends
“Expanding Clinical Applications and Pipeline Innovation”
- A significant and accelerating trend in the global RNA Interference (RNAi) Nucleic Acid Therapeutics market is the rapid expansion of clinical applications, particularly in treating rare genetic diseases, oncology, and metabolic disorders. With the success of FDA-approved RNAi therapies such as Onpattro (for hereditary ATTR amyloidosis) and Givlaari (for acute hepatic porphyria), pharmaceutical companies are increasingly investing in broadening the therapeutic scope of RNAi technologies
- For instance, companies such as Alnylam Pharmaceuticals, Arrowhead Pharmaceuticals, and Dicerna Pharmaceuticals are aggressively expanding their RNAi pipelines, targeting liver, CNS, and cardiovascular diseases. Multiple candidates are progressing into late-stage trials, with promising efficacy and safety profiles fueling investor and clinical interest
- Innovations in RNA chemistry and delivery platforms—such as GalNAc conjugation for targeted liver delivery—are enhancing the specificity, potency, and durability of RNAi therapies. These advancements significantly reduce dosing frequency and improve patient compliance, making RNAi drugs more viable for chronic treatment settings
- In addition, collaborations between biotech firms and global pharma giants are accelerating commercialization and global market entry of RNAi therapies. These partnerships are providing essential funding, infrastructure, and distribution channels to bring therapies to market faster and at scale
- This trend toward expanding RNAi applications and therapeutic areas is fundamentally reshaping the biopharmaceutical landscape, enabling the development of precision therapies for conditions previously deemed untreatable. The growing number of investigational new drug (IND) filings and regulatory designations underscore the momentum driving this field forward
- The demand for RNAi-based therapies is growing rapidly across both developed and emerging healthcare markets, as clinicians and patients increasingly prioritize targeted, disease-modifying treatments with long-term efficacy and fewer off-target effects
RNA Interference (RNAi) Nucleic Acid Therapeutics Market Dynamics
Driver
“Growing Need Due to Rising Prevalence of Genetic and Rare Diseases”
- The increasing prevalence of genetic, metabolic, and rare diseases, coupled with the urgent demand for highly targeted therapeutic approaches, is a significant driver for the growing adoption of RNA Interference (RNAi) Nucleic Acid Therapeutics
- For instance, in April 2024, Alnylam Pharmaceuticals announced the expansion of its RNAi pipeline with a new investigational siRNA therapy targeting complement-mediated diseases. Such developments by leading players are expected to accelerate market growth during the forecast period
- As patients and healthcare providers seek innovative treatment options beyond conventional therapies, RNAi drugs provide a precise mechanism to silence disease-causing genes, offering a major advancement in personalized medicine
- Furthermore, the growing focus on rare diseases, supported by regulatory incentives such as Orphan Drug Designation and Fast Track approval, is making RNAi a preferred modality for drug development, particularly in underserved therapeutic areas
- The convenience of infrequent dosing, strong safety profiles, and expanding delivery technologies (such as GalNAc conjugates for liver targeting) are key factors propelling the adoption of RNAi therapeutics in both hospital and outpatient settings. The trend toward patient-centric, long-acting therapies and the increasing availability of user-friendly RNAi treatment options further contribute to global market growth
Restraint/Challenge
“Delivery Limitations and High Development Costs”
- Despite their therapeutic potential, challenges surrounding effective delivery of RNAi molecules to tissues beyond the liver pose a significant barrier to broader application. Most approved RNAi drugs currently rely on liver-targeted delivery, limiting their use for diseases in other organs such as the brain, lungs, or kidneys
- For instance, while siRNA delivery using GalNAc conjugates is highly effective for hepatocytes, efforts to develop delivery systems for extrahepatic tissues remain in early stages and face numerous technical and biological hurdles
- Addressing these delivery challenges through lipid nanoparticles (LNPs), polymers, and novel conjugates is critical for expanding the RNAi drug pipeline and reaching broader therapeutic areas
- In addition, the high initial cost of RNAi drug development, including synthesis, stability testing, and clinical trials, can deter smaller biotech firms from entering the space
- While funding and strategic partnerships are helping mitigate cost pressures, the perceived high investment risk, especially in early-stage programs, remains a concern for investors and developers
- Overcoming these obstacles through innovative delivery technologies, improved scalability, and regulatory support for novel mechanisms will be vital for sustaining long-term market expansion
RNA Interference (RNAi) Nucleic Acid Therapeutics Market Scope
The market is segmented on the basis of type, route of administration, indication, end user, and application.
- By Type
On the basis of type, the RNA Interference (RNAi) nucleic acid therapeutics market is segmented into small interfering RNA (siRNA) and MicroRNA (miRNA). The small interfering RNA (siRNA) segment held the largest market revenue share of 61.3% in 2024, owing to its advanced therapeutic development stage, higher clinical success rates, and broader application in rare genetic diseases and liver-based disorders. siRNA-based therapies such as Onpattro and Givlaari have already received regulatory approval, driving commercial momentum.
The miRNA segment is projected to witness the fastest growth rate from 2025 to 2032, driven by its emerging role in modulating gene expression in cancer and metabolic disorders, with expanding research initiatives and early-phase clinical trials showing encouraging outcomes.
- By Route of Administration
On the basis of route of administration, the market is segmented into Intravenous, intradermal, pulmonary delivery, intrathecal, and others. The intravenous segment held the largest share in 2024, due to its established use in delivering siRNA therapeutics directly into systemic circulation, particularly in clinical settings.
The pulmonary delivery segment is anticipated to exhibit the fastest CAGR during the forecast period, as research advances in inhalation-based delivery systems promise more targeted and non-invasive RNAi therapy for respiratory diseases.
- By Indication
On the basis of indication, the market is segmented into acute hepatic porphyria (AHP), hereditary transthyretin-mediated amyloidosis (hATTR), hypercholesterolemia, and others. The hereditary transthyretin-mediated amyloidosis (hATTR) segment accounted for the highest market revenue in 2024, supported by commercial success of RNAi therapies such as Onpattro and Amvuttra.
The hypercholesterolemia segment is forecasted to grow at the highest CAGR from 2025 to 2032, driven by the promising clinical performance of investigational RNAi therapies targeting PCSK9 and other lipid-related pathways.
- By End User
On the basis of end user, the market is segmented into hospitals & clinics, research & academic institutes, pharmaceutical & biotechnology companies, and others. The hospitals & clinics segment held the dominant revenue share in 2024, given the current administration of RNAi therapies under clinical supervision and the management of rare diseases in specialty centers.
The pharmaceutical & biotechnology companies segment is expected to grow at the highest CAGR, owing to heightened investment in RNAi R&D, expanding pipelines, and strategic partnerships aimed at drug development and commercialization.
- By Application
On the basis of application, the market is segmented into genetic disorders, oncology, neurodegenerative disorders, infectious diseases, and others. The genetic disorders segment led the market in 2024, due to the high unmet need in rare genetic diseases and the precision targeting capability of RNAi.
Oncology is projected to be the fastest-growing application segment, driven by the increasing use of RNAi to silence cancer-causing genes and ongoing clinical trials exploring its potential in solid tumors and hematologic malignancies.
RNA Interference (RNAi) Nucleic Acid Therapeutics Market Regional Analysis
- North America dominated the RNA Interference (RNAi) nucleic acid therapeutics market with the largest revenue share of 45.7% in 2024, primarily due to a strong presence of leading biotechnology and pharmaceutical companies, advanced healthcare infrastructure, and significant investment in RNA-based drug development
- The region's leadership is reinforced by active clinical trials, favorable regulatory support from the FDA, and successful commercialization of RNAi therapies such as Onpattro and Givlaari
- Moreover, North America benefits from robust academic and research collaborations, government funding for rare disease treatments, and increasing prevalence of genetic disorders and metabolic conditions, further accelerating the adoption of RNAi-based therapeutics across both clinical and research settings
U.S. RNA Interference (RNAi) Nucleic Acid Therapeutics Market Insight
The U.S. RNA Interference (RNAi) Nucleic Acid Therapeutics market captured the largest revenue share within North America in 2024. This dominance is fueled by a robust biotechnology and pharmaceutical industry, significant investments in R&D, and a favorable regulatory environment that supports the development and approval of novel RNAi therapies. Consumers (patients and healthcare providers) are increasingly prioritizing advanced, targeted treatments for genetic disorders, rare diseases, and chronic conditions where RNAi therapeutics offer a promising solution. The growing preference for precision medicine and the integration of cutting-edge genomic and molecular research further propels the RNA Interference (RNAi) Nucleic Acid Therapeutics industry. Moreover, the increasing number of FDA-approved RNAi drugs and a strong pipeline of candidates significantly contribute to the market's expansion.
Europe RNA Interference (RNAi) Nucleic Acid Therapeutics Market Insight
The Europe RNA Interference (RNAi) Nucleic Acid Therapeutics market is projected to expand at a substantial CAGR from 2025 to2032 This growth is primarily driven by increasing awareness of RNAi technology's therapeutic potential, rising prevalence of chronic and genetic diseases, and a strong focus on advanced healthcare solutions across the region. The increase in healthcare expenditure and robust research infrastructure are fostering the adoption of RNAi therapies. European healthcare systems are drawn to the targeted nature and efficacy these therapies offer in treating previously intractable diseases. The region is experiencing significant growth driven by continued investment in biotech research, favorable government support for innovative medicines, and expanding patient access to cutting-edge treatments.
U.K. RNA Interference (RNAi) Nucleic Acid Therapeutics Market Insight
The U.K. RNA Interference (RNAi) Nucleic Acid Therapeutics market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by the escalating research and development activities in genomics and gene-silencing technologies, coupled with a strong emphasis on personalized medicine. In addition, the high burden of genetic and chronic diseases is encouraging investment in novel therapeutic solutions. The UK’s robust life sciences sector, alongside its strong academic research institutions and a supportive regulatory framework, is expected to continue to stimulate market growth.
Germany RNA Interference (RNAi) Nucleic Acid Therapeutics Market Insight
The Germany RNA Interference (RNAi) Nucleic Acid Therapeutics market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing awareness of advanced biopharmaceuticals and the demand for highly targeted, innovative treatment options. Germany’s well-developed healthcare infrastructure, combined with its strong pharmaceutical industry and emphasis on medical innovation, promotes the adoption of RNAi therapeutics, particularly in specialized clinics and research hospitals. The integration of RNAi therapies into personalized medicine approaches and a strong preference for high-quality, evidence-based treatments align with local healthcare expectations.
Asia-Pacific RNA Interference (RNAi) Nucleic Acid Therapeutics Market Insight
The Asia-Pacific RNA Interference (RNAi) Nucleic Acid Therapeutics market is poised to grow at the fastest CAGR, estimated at over 17.4% during the forecast period of 2025 to 2032. This rapid growth is driven by increasing healthcare expenditure, rising prevalence of chronic and genetic disorders, and significant advancements in biotechnology and pharmaceutical R&D in countries such as China, Japan, and India. The region's growing investment in modern healthcare facilities, supported by government initiatives promoting pharmaceutical innovation and access to advanced therapies, is driving the adoption of RNAi therapeutics. Furthermore, as APAC emerges as a manufacturing hub for biologics and a key market for rare disease treatments, the affordability and accessibility of RNAi options are expanding to a wider patient base.
China RNA Interference (RNAi) Nucleic Acid Therapeutics Market Insight
The China RNA Interference (RNAi) Nucleic Acid Therapeutics market accounted for the largest market revenue share in Asia Pacific in 2024, attributed to the country's rapidly expanding healthcare sector, massive patient population, and high rates of technological adoption in medicine. China is increasingly becoming a hub for biotechnological innovation, with strong domestic pharmaceutical companies investing heavily in RNAi research and development. The push towards modernizing healthcare, coupled with government support for local drug development and increasing access to advanced therapies, are key factors propelling the market in China.
India RNA Interference (RNAi) Nucleic Acid Therapeutics Market Insight
The India RNA Interference (RNAi) Nucleic Acid Therapeutics market is anticipated to grow at a noteworthy CAGR from 2025 to 2032 This growth is primarily driven by the increasing burden of genetic and chronic diseases, growing awareness about RNAi-based therapies, and significant advancements in India's biotechnology and pharmaceutical sectors. Government initiatives to improve healthcare access and foster domestic drug manufacturing, along with rising investments in R&D by both local and international players, are accelerating market expansion. The expanding patient pool and the push for innovative, targeted treatments are encouraging the adoption of RNAi therapeutics in India.
RNA Interference (RNAi) Nucleic Acid Therapeutics Market Share
The RNA Interference (RNAi) Nucleic Acid Therapeutics industry is primarily led by well-established companies, including:
- Alnylam Pharmaceuticals, Inc. (U.S.)
- Ionis Pharmaceuticals, Inc. (U.S.)
- Arrowhead Pharmaceuticals, Inc. (U.S.)
- Silence Therapeutics (U.K.)
- Arcturus Therapeutics, Inc. (U.S.)
- Sirnaomics (U.S.)
- OliX Pharmaceuticals, Inc. (South Korea)
- Arbutus Biopharma (U.S.)
- Vir Biotechnology, Inc. (U.S.)
- Wave Life Sciences (U.S.)
- Switch Therapeutics (U.S.)
Latest Developments in Global RNA Interference (RNAi) Nucleic Acid Therapeutics Market
- In March 2025, Alnylam Pharmaceuticals, Inc. received FDA approval for AMVUTTRA (vutrisiran) in treating transthyretin-mediated cardiomyopathy (ATTR‑CM), making it the first RNAi therapeutic targeting both cardiomyopathy and polyneuropathy in amyloidosis. This significant milestone is expected to expand the therapy’s market and improve patient access
- In March 2025, Alnylam's Qfitlia (fitusiran), an siRNA therapeutic for hemophilia A and B (with or without inhibitors), received FDA approval, becoming the first RNAi drug to directly reduce bleeding rates in hemophilia patients
- In February 2025, At its R&D Day in New York City, Alnylam announced progress on multiple next-generation RNAi candidates, including its nucresiran Phase 3 trial (TRITON) targeting ATTR‑CM, alongside advancements in cardiovascular and neuroscience pipelines
- In January 2025, Alnylam reported record net product revenues of USD 1.646 billion for 2024 (33% YoY growth), driven by strong sales of Onpattro, Amvuttra, Givlaari, and Oxlumo, and guided to over USD 2 billion in 2025
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












