North America Nerve Repair And Regeneration Market
Market Size in USD Billion
CAGR :
% 
 USD
3.04 Billion
USD
7.31 Billion
2025
2033
USD
3.04 Billion
USD
7.31 Billion
2025
2033
| 2026 –2033 | |
| USD 3.04 Billion | |
| USD 7.31 Billion | |
|
|
|
|
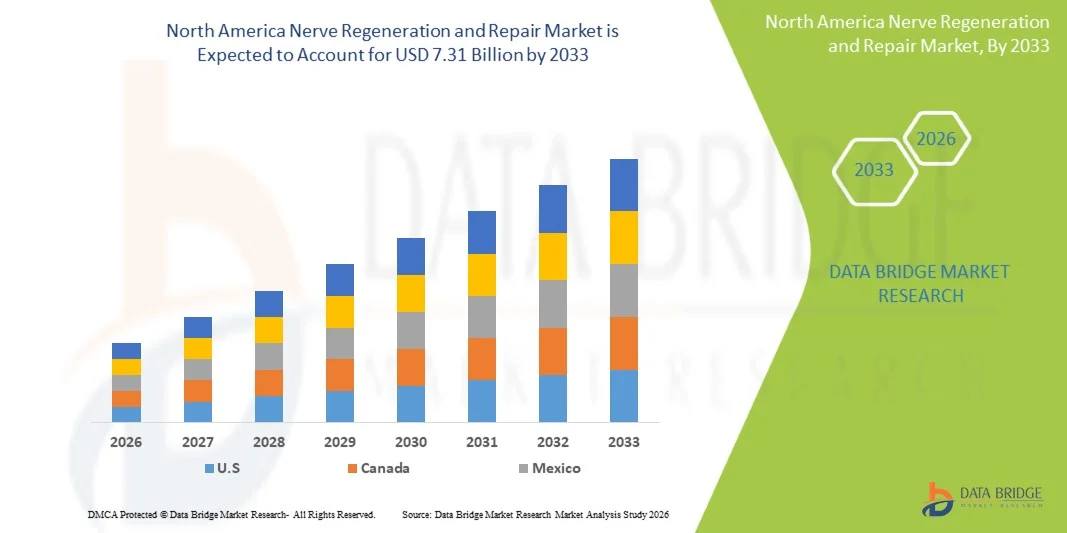
North America Nerve Regeneration and Repair Market Size
- The North America Nerve Regeneration and Repair Market size was valued at USD 3.04 billion in 2025 and is expected to reach USD 7.31 billion by 2033, at a CAGR of11.60% during the forecast period
- The market growth is largely fueled by the growing adoption and technological progress within regenerative medicine, advanced biomaterials, and neurotechnology, leading to improved treatment options for nerve injuries and neurological disorders
- Furthermore, rising patient demand for minimally invasive, effective, and personalized solutions for nerve repair and regeneration is establishing advanced nerve regeneration products as a critical choice in both clinical and research settings. These converging factors are accelerating the uptake of Nerve Regeneration and Repair solutions, thereby significantly boosting the industry's growth
North America Nerve Regeneration and Repair Market Analysis
- Nerve regenerative therapies, nerve conduits, and biomaterial-based scaffolds are increasingly vital in treating nerve injuries and neurological disorders in both clinical and research settings due to their effectiveness, precision, and ability to restore nerve function
- The escalating demand for advanced nerve repair solutions is primarily fueled by rising prevalence of peripheral nerve injuries, increasing geriatric population, and growing preference for minimally invasive, patient-specific therapies
- U.S. dominated the North America Nerve Regeneration and Repair Market with the largest revenue share of 38% in 2025, characterized by advanced healthcare infrastructure, high R&D investment, and a strong presence of key industry players. The U.S. experienced substantial growth in nerve regeneration procedures, driven by innovations in biomaterials, nerve conduits, and stem-cell-based therapies
- Canada is expected to be the fastest growing region in the North America Nerve Regeneration and Repair Market during the forecast period, with a CAGR of 12%, due to increasing incidence of nerve injuries, expanding healthcare infrastructure, and rising adoption of advanced regenerative therapies
- The Biomaterials segment dominated with a 47.1% revenue share in 2025, attributed to widespread use in nerve scaffolding, conduits, and grafting procedures
Report Scope and North America Nerve Regeneration and Repair Market Segmentation
|
Attributes |
Nerve Regeneration and Repair Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
North America Nerve Regeneration and Repair Market Trends
“Enhanced Convenience Through AI and Voice Integration”
- A significant and accelerating trend in the North America Nerve Regeneration and Repair Market is the growing adoption of advanced regenerative therapies, including stem cell therapy, tissue engineering, and nerve guidance conduits, aimed at improving functional recovery for peripheral and central nerve injuries
- For instance, in March 2024, AxoGen, Inc. launched an enhanced Avance Nerve Graft designed to promote faster and more effective nerve repair in patients with traumatic nerve injuries
- Increasing investment in bioengineered scaffolds, growth factors, and 3D bioprinting technologies is enabling more precise nerve regeneration and rehabilitation outcomes
- Collaboration between biotech companies, research institutions, and hospitals is accelerating innovation and the commercialization of new nerve repair solutions
- Growing awareness among healthcare providers and patients about early intervention and advanced rehabilitation techniques is reshaping treatment approaches
- The trend toward combination therapies, integrating surgical repair with pharmacological and regenerative approaches, is expected to further enhance clinical outcomes in nerve regeneration
North America Nerve Regeneration and Repair Market Dynamics
Driver
“Growing Need Due to Rising Incidence of Nerve Injuries and Neurological Disorders”
- The increasing prevalence of nerve injuries due to trauma, surgeries, diabetes, and neuropathic disorders is driving demand for advanced regenerative therapies
- For instance, according to the 2023 WHO report, over 1.2 million people worldwide suffer from peripheral nerve injuries annually, emphasizing the urgent need for innovative repair solutions
- Rising geriatric population and associated nerve degeneration disorders are contributing to higher adoption of regenerative therapies
- Expanding healthcare infrastructure and specialized nerve repair centers in North America and Europe are facilitating treatment access
- Increased funding from governments and private institutions for research and clinical trials is accelerating product development
- Growing patient awareness about the benefits of early intervention and regenerative therapies is enhancing market penetration
Restraint/Challenge
“High Cost, Regulatory Hurdles, and Safety Concerns”
- The high cost of advanced regenerative therapies and complex regulatory approval processes pose significant challenges for market growth
- For instance, delays in the approval and commercialization of certain stem cell-based nerve repair treatments in the European Union highlight regulatory complexities
- Limited long-term clinical data and variability in treatment outcomes create hesitancy among healthcare providers
- Technical challenges in scaling the production of bioengineered scaffolds and stem cell therapies may lead to supply constraints
- Safety concerns, including immune rejection and potential complications in cell-based therapies, can hinder adoption
- Competition from conventional treatments such as physical therapy and synthetic nerve conduits may slow overall market growth
North America Nerve Regeneration and Repair Market Scope
The market is segmented on the basis of surgery, product, and end-user.
• By Surgery
On the basis of surgery, the market is segmented into Direct Neuropathy, Nerve Grafting, Stem Cell Therapy, and Neuromodulation Surgery. The Stem Cell Therapy segment dominated the largest market revenue share of 45.2% in 2025, driven by its established efficacy in promoting nerve regeneration and functional recovery. Stem cell therapies are increasingly adopted for peripheral nerve injuries and complex neuropathies. Hospitals and specialized neurology centers favor stem cell interventions due to reduced complications and improved patient outcomes. Advanced processing and delivery systems enhance precision and therapeutic effectiveness. Ongoing clinical trials validate safety and efficacy, further encouraging adoption. Integration with rehabilitation programs improves patient recovery timelines. Expansion of regenerative medicine centers ensures therapy availability. Reimbursement coverage in key markets supports accessibility. Patient preference for minimally invasive solutions strengthens demand. Government funding for regenerative therapy research enhances pipeline innovation. Collaboration with academic and research institutions accelerates development. These factors collectively maintain stem cell therapy as the leading surgical segment in 2025.
The Neuromodulation Surgery segment is expected to witness the fastest CAGR of 13.6% from 2026 to 2033, driven by growing adoption of implantable devices and electrical stimulation therapies for chronic neuropathic pain and functional disorders. Hospitals and ambulatory surgical centers increasingly implement neuromodulation for pain management and nerve repair. Technological advancements in electrode design and programmable devices improve patient outcomes. Integration with digital monitoring platforms supports long-term therapy tracking. Rising prevalence of peripheral neuropathies and diabetic neuropathy accelerates demand. Outpatient and minimally invasive procedures enhance patient convenience. Increased physician awareness and specialized training boost adoption. Reimbursement policies for neuromodulation devices expand market accessibility. Clinical evidence supports pain reduction and improved quality of life, fueling confidence. Government initiatives for advanced pain management technologies support expansion. Rising patient preference for non-pharmacological therapies strengthens uptake. Expansion of private clinics offering neuromodulation drives regional penetration. These drivers collectively position neuromodulation surgery as the fastest-growing segment.
• By Product
On the basis of product, the market is segmented into Biomaterials and Neuromodulation Surgery Devices. The Biomaterials segment dominated with a 47.1% revenue share in 2025, attributed to widespread use in nerve scaffolding, conduits, and grafting procedures. Hospitals and neurology centers prioritize biomaterials for their compatibility, safety, and regenerative potential. Advanced polymer and hydrogel technologies enhance functional recovery. Strong clinical evidence supports consistent nerve repair outcomes. Integration with surgical protocols improves precision and standardization. Expansion of regenerative medicine research and production capabilities ensures supply reliability. Reimbursement schemes support high-cost biomaterial adoption. Continuous innovation in material science enhances durability and patient safety. Training programs for surgical teams strengthen device utilization. Collaboration with biotech firms accelerates product pipeline development. Patient preference for minimally invasive and implantable solutions reinforces adoption. These factors collectively maintain biomaterials as the dominant product segment in 2025.
The Neuromodulation Surgery Devices segment is expected to witness the fastest CAGR of 12.9% from 2026 to 2033, driven by technological innovation in implantable stimulators and wearable therapy devices. Hospitals and outpatient centers are rapidly integrating these devices for pain management, neuropathic conditions, and rehabilitation. Advances in programmable and wireless devices enhance precision therapy. Digital connectivity enables remote monitoring and improved clinical outcomes. Rising prevalence of chronic neuropathies and post-surgical nerve injuries fuels demand. Regulatory approvals in key regions support adoption. Patient preference for non-invasive and reversible treatments strengthens uptake. Physician training and specialized workshops boost confidence in device use. Integration with rehabilitation programs enhances therapy effectiveness. Reimbursement coverage for neuromodulation devices expands patient access. Clinical research demonstrates pain reduction and quality-of-life improvements. Expansion of private clinics and outpatient centers ensures regional penetration. These combined drivers position neuromodulation devices as the fastest-growing product segment.
• By End-User
On the basis of end-user, the market is segmented into Hospitals, Neurology Clinics, Home Healthcare, and Community Healthcare. The Hospitals segment dominated with a 51.6% revenue share in 2025, driven by advanced infrastructure, availability of specialized neurology and regenerative medicine teams, and high patient volumes for complex nerve repair procedures. Hospitals maintain surgical units equipped for stem cell therapy, biomaterials implantation, and neuromodulation surgeries. Integration with rehabilitation and diagnostic services ensures comprehensive care. Favorable reimbursement structures and insurance coverage support procurement. Hospitals participate in clinical trials and research collaborations, ensuring early adoption of novel therapies. Investment in ICU and monitoring facilities enables safe treatment delivery. High-volume surgical programs and post-operative care facilities enhance revenue. Patient preference for hospital-based care strengthens adoption. Digital workflow integration improves clinical efficiency and outcome tracking. Long-term treatment follow-up supports therapy success. Government initiatives supporting advanced neurological care reinforce hospital dominance. These factors collectively maintain hospitals as the leading end-user segment in 2025.
The Home Healthcare segment is projected to witness the fastest CAGR of 14.2% from 2026 to 2033, driven by increasing patient preference for minimally invasive and home-based post-surgical recovery solutions. Portable biomaterials, stem cell kits, and neuromodulation devices enable home use. Telemedicine integration allows remote monitoring and virtual follow-up with clinicians. Aging populations and chronic neuropathic conditions increase demand. Cost-effectiveness and reduced hospital visits favor adoption. Homecare services expand access to rehabilitation programs. Rising awareness among patients and caregivers supports utilization. Insurance coverage and government initiatives for home-based care improve market penetration. Integration with wearable monitoring devices enhances therapy adherence. Training programs for caregivers ensure safe and effective administration. Convenience and comfort of home recovery encourage patient uptake. Expansion of private home healthcare providers strengthens regional reach. These drivers collectively position home healthcare as the fastest-growing end-user segment.
North America Nerve Regeneration and Repair Market Regional Analysis
- North America dominated the North America Nerve Regeneration and Repair Market with the largest revenue share of 38% in 2025, characterized by advanced healthcare infrastructure, high R&D investment, and a strong presence of key industry players
- The region experienced substantial growth in nerve regeneration procedures, driven by innovations in biomaterials, nerve conduits, and stem-cell-based therapies
- Consumers in the region are increasingly prioritizing advanced treatment options, minimally invasive procedures, and improved functional recovery, which is driving the adoption of both surgical and non-surgical nerve repair solutions
U.S. North America Nerve Regeneration and Repair Market Insight
The U.S. North America Nerve Regeneration and Repair Market dominated the North America Nerve Regeneration and Repair Market with the largest revenue share of 38% in 2025, fueled by advanced healthcare infrastructure, high R&D investment, and the presence of leading global nerve repair companies. The country witnessed significant growth in the adoption of biomaterials, neuromodulation devices, and stem-cell-based therapies for peripheral and central nerve injuries. The increasing prevalence of traumatic nerve injuries, combined with innovations in regenerative medicine, is accelerating market expansion and driving improved patient outcomes.
Canada North America Nerve Regeneration and Repair Market Insight
Canada North America Nerve Regeneration and Repair Market is expected to be the fastest-growing region in the North America Nerve Regeneration and Repair Market during the forecast period, with a CAGR of 12%, driven by rising incidence of nerve injuries, expanding healthcare infrastructure, and growing adoption of advanced regenerative therapies. The country is witnessing increased investments in research, clinical trials, and integration of cutting-edge biomaterials and neuromodulation devices into healthcare settings, supporting market growth across hospitals, clinics, and specialized nerve care centers.
North America Nerve Regeneration and Repair Market Share
The Nerve Regeneration and Repair industry is primarily led by well-established companies, including:
- Axogen (U.S.)
- Medtronic (U.S.)
- Integra LifeSciences (U.S.)
- Stryker (U.S.)
- Boston Scientific (U.S.)
- Zimmer Biomet (U.S.)
- NeuroMetrix (U.S.)
- Cook Medical (U.S.)
- Polyganics (Netherlands)
- NervGen (Canada)
- RTI Surgical (U.S.)
- Nuo Therapeutics (U.S.)
- Globus Medical (U.S.)
- Axonics Modulation Technologies (U.S.)
- ReNeuron (U.K.)
- Acelity (U.S.)
- B. Braun (Germany)
- Pfizer (U.S.)
- Novartis (Switzerland)
- Teva Pharmaceuticals (Israel)
Latest Developments in North America Nerve Regeneration and Repair Market
- In June 2024, AxoGen launched Avive+ Soft Tissue Matrix™, a new multi‑layer amniotic membrane allograft designed to support nerve repair procedures by acting as a soft‑tissue barrier that protects and separates regenerating nerves from surrounding tissues, expanding its peripheral nerve repair portfolio
- In April 2025, Orthocell Limited received FDA 510(k) clearance for Remplir, its collagen nerve wrap designed for surgical nerve repair. Clearance enables its marketing in the U.S. and represents a key commercial milestone for biomaterial‑based nerve regeneration products
- In June 2025, ReNerve entered a strategic partnership with Berkeley Biologics LLC to develop and commercialize two new tissue‑based product ranges (human dermal and amniotic tissues) that complement its existing nerve repair device portfolio, enhancing its market offerings for reconstructive and trauma‑related nerve procedures
- In May 2025, NervGen Pharma announced that it would present topline data from its Phase 1b/2a clinical trial of NVG‑291 (a novel neuroreparative agent for spinal cord injury) at the American Spinal Injury Association Annual Scientific Meeting, demonstrating progress in clinical evaluation of therapies targeting nerve regeneration
- In August 2025, NervGen Pharma reported positive preclinical results for NVG‑291‑R (a rodent analogue of NVG‑291) showing significant functional recovery and axonal regeneration in models of peripheral nerve injury and blast‑induced sensorineural hearing loss, underscoring broader regenerative potential across nerve repair indications
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.