North America Ovarian Cancer Diagnostics Market
Market Size in USD Billion
CAGR :
% 
 USD
3,124.06 Billion
USD
5,675.36 Billion
2022
2030
USD
3,124.06 Billion
USD
5,675.36 Billion
2022
2030
| 2023 –2030 | |
| USD 3,124.06 Billion | |
| USD 5,675.36 Billion | |
|
|
|
|
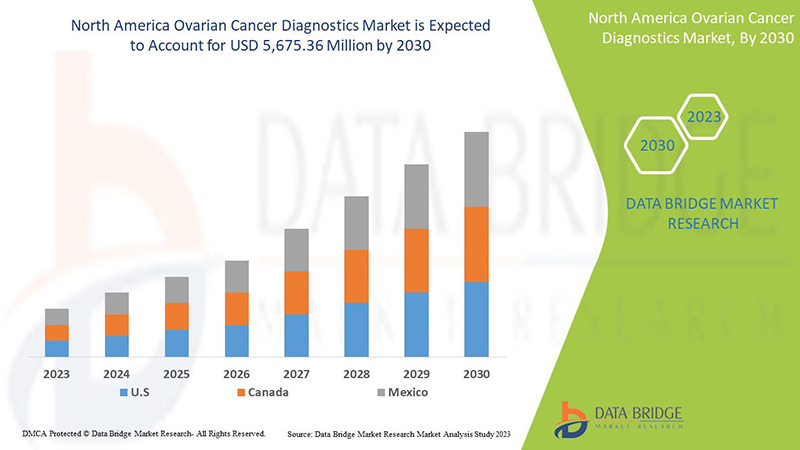
North America Ovarian Cancer Diagnostics Market Analysis and Insights
Ovarian cancer is the type of cancer that forms in tissues of the ovary (one of a pair of female reproductive glands in which the ova, or eggs, are formed). Most ovarian cancers are either ovarian epithelial cancers (cancer that begins in the cells on the surface of the ovary) or malignant germ cell tumors (cancer that begins in egg cells). The tests and procedures used to diagnose ovarian cancer includes the pelvic exam, imaging tests, blood tests, surgery and among others. During a pelvic exam, the doctor inserts gloved fingers into the vagina and simultaneously presses a hand on the abdomen in order to feel (palpate) the pelvic organs.
North America ovarian cancer diagnostics market is expected to grow in the forecast period of 2023 to 2030. Data Bridge Market Research analyses that the market is growing with a CAGR of 7.8% in the forecast period of 2023 to 2030 and is expected to reach USD 5,675.36 million by 2030 from USD 3,124.06 million in 2022.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customisable to 2020-2016) |
|
Quantitative Units |
Revenue in USD Million |
|
Segments Covered |
By Product Type (Instruments, Kits and Reagents), Procedure Type (Biopsy Test, Medical Imaging Test, Blood Markers Testing and Genetic Testing), Cancer Type (Germ Cell, Epithelial Tumor and Stromal Cell Tumor), End User (Cancer Diagnostic Centers, Hospital Laboratories, Research Institutes and Others) |
|
Countries Covered |
U.S., Canada and Mexico |
|
Market Players Covered |
F. Hoffmann-La Roche Ltd, Tosoh India Pvt. Ltd., Luminex Corporation, Quest Diagnostics Incorporated, Thermo Fisher Scientific Inc., Ngenebio, Abbott, Siemens healthcare private limited, Myriad genetics Inc., Bio-rad laboratories, Inc., R&d systems, Inc., Foundation medicine, Inc., Biosupply ltd, Lcm genect srl, Inex innovate private limited, Abcam plc., Monobind Inc., Fujirebio, Mp biomedicals, Biovision Inc., Boster biological technology, Biogenix Inc. Pvt. Ltd., Genway biotech and Lifespan biosciences, Inc. |
Market Definition of North America Ovarian Cancer Diagnostics Market
Ovarian cancer is most common in women between the ages of 50 and 79. It is becoming more prevalent as the world's geriatric population grows and there is a greater emphasis on early detection and treatment, which is expected to accelerate ovarian cancer diagnostic market development. Increasing government investment in raising awareness about early cancer detection as well as increasing health-care spending would also propel business growth. Obesity appears to play a significant role in the development of ovarian cancer. Other lifestyle choices that can raise the risk include smoking, drinking, and not having children. Since ovarian cancer is not easily detectable, women who are at risk of developing the disease must undergo routine testing to identify the disease early, allowing the market to expand.
The North America ovarian cancer diagnostic market is growing in the forecast year due to the rise in market players and the availability of advanced services. Along with this, manufacturers are engaged in R&D activity for launching novel services in the market. The increasing research in ovarian cancer diagnostics and development is further boosting the market growth. However, difficulties in ovarian cancer screening techniques might hamper the growth of the North America ovarian cancer diagnostic market in the forecast period.
North America Ovarian Cancer Diagnostics Market Dynamics
Drivers
- Growing Ovarian Cancer Awareness
Growing ovarian cancer awareness has led to an increased demand for timely cancer detection, leading to market growth.
Ovarian cancer is one of the major causes of rising mortality rates among female populations worldwide, which is fuelling market growth over the next five years. Cancer in the ovaries and cysts is becoming more common due to various factors such as environmental factors and genetic mutations.
Ovarian cancer is a type of cancer that affects women’s egg-producing organs, the ovaries. Ovarian cancer is difficult to diagnose because the symptoms are vague and are often detected only after cancer has spread through the stomach and pelvis, making it difficult to cure.
As a result, improved diagnostic processes and techniques are required to determine the cancer stage to treat. Furthermore, the rising mortality rate from ovarian cancer is concerning, emphasizing the importance of early detection so that treatment can be provided.
Due to an increase in awareness about ovarian cancer, it is expected to act as a driving factor for market growth.
- Improved Diagnostic Processes and Techniques
Screening tests and exams are used to detect a disease, such as cancer, in people who do not have any symptoms. There has been a lot of research to develop a screening test for ovarian cancer, but there has not been much success so far. The two tests used most often (in addition to a complete pelvic exam) to screen for ovarian cancer are Transvaginal Ultrasound (TVUS) and the CA-125 blood test.
TVUS is a test that uses sound waves to look at the uterus, fallopian tubes, and ovaries by putting an ultrasound wand into the vagina. It can help find a mass (tumor) in the ovary, but it cannot actually tell if a mass is cancer or benign. When it is used for screening, most of the masses found are not cancer.
The CA-125 blood test measures the amount of a protein called CA-125 in the blood. Many women with ovarian cancer have high levels of CA-125. This test can be useful as a tumor marker to help guide treatment in women known to have ovarian cancer because a high level often goes down if treatment is working. But checking CA-125 levels is not as useful as a screening test for ovarian cancer.
Thus, due to the increase in improved diagnostic processes and techniques, it is expected to act as a driving factor for market growth.
RESTRAINTS
High Cost of Diagnosis
Worldwide, the costs of cancer treatment have increased. Health industries are facing several challenges like medical costs for cancer care. The cost of cancer care in 2010 was USD 124.60 billion which was projected to increase to USD 173.00 billion by 2020 with cancer drug prices and acute hospital care as the main drivers. Hence, the increased cost of diagnostic agents production is hampering the market growth.
Lack of Skilled Professionals
Healthcare professionals involved in the diagnostic process have an obligation and ethical responsibility to employ clinical reasoning skills, evaluate, and manage a patient's medical problems. When a diagnosis is accurate and made in a timely manner, a patient has the best opportunity for a positive health outcome because clinical decision-making will be tailored to a correct understanding of the patient's health problem. The lack of skilled professionals may hinder the recovery process of the patient and thus, may hamper the market growth.
OPPORTUNITIES
Increasing Healthcare Expenditure for Cancer Diagnosis and Treatment
Across the globe, R&D activities are escalating owing to public health expenditure with economic performances whereas, the healthcare industry ranks second among all industries when it comes to the amount spent on healthcare. Rising healthcare expenditure can result in better provision of R&D opportunities. It is anticipated to upsurge the demand for ovarian cancer diagnostics.
Increasing healthcare expenditure for cancer treatment also helps patients to take hassle-free advanced diagnostics and treatment for fast recovery. The spending on healthcare is made up of the combination of out-of-pocket payments (people paying for their own care), government expenditure, and sources including health insurance and activities by Non-Governmental Organizations (NGOs). Due to this increasing healthcare expenditure for cancer treatment, it acts as an opportunity for market growth.
CHALLENGES
Strict Regulations and Standards for the Approval and Commercialization of Cancer Diagnostic Products
The stringent regulations for commercialization of any product in the market are proving to be a big challenge for manufacturers of cancer diagnostic products in the U.S. and European region. Every country has its own regulations and has a different body for regulatory procedures.
In the U.S., manufacturers require marketing authorization approval for IVD products for human use. The product must be labeled in accordance with labeling regulations. Establishments involved in the production and distribution of medical devices intended for commercial distribution in the U.S. are required to register with the FDA. Registration provides the FDA with the location of medical device manufacturing facilities and importers. Registration of an establishment is not an approval of the establishment or its devices by the FDA, that is, it does not provide FDA authorization to market the device. Unless exempted, premarketing authorization is required before a device can be placed into commercial distribution in the U.S.
The regulatory requirement for approvals of marketing as well as the declaration of conformity and the time required for regulatory review may vary for different products. The company which fails to get regulatory approval harms the business because without getting approval on the products, manufacturers are not able to launch their product in the market and for this reason, strict regulations and standards for the approval and commercialization of cancer diagnostic products act as a restraining factor for market growth.
Recent Developments
- In November 2022, Myriad genetics Inc. announced that it has acquired Gateway Genomics, LLC, The acquisition strengthens Myriad Genetics’ portfolio of Women’s Health products, expanding access to personalized genetic tests during the reproductive stage of a women’s life and beyond. With SneakPeek, Myriad now serves women earlier in their pregnancy, providing data-driven genetic insights through their lifetime with the Prequel non-invasive prenatal screen, Foresight carrier screen, and MyRisk Hereditary Cancer Test with Risk Score for all ancestries, this will help the company to increase their revenue.
- In October 2022, Quest Diagnostics announced the new phase of collaboration with Decode health, in the starting phase of collaboration, the two parties developed RNA (transcriptome) sequencing capabilities based on both parties' next-generation sequencing, analytics and clinical expertise. The collaboration is significant as biomarker-based data can help reduce the time and cost of developing novel diagnostic tests and identifying new drug targets for different types of cancers (breast, prostate and ovarian cancer). This collaboration helps the company to find innovative paths in the field of R&D and increases the North America presence of the company.
North America Ovarian Cancer Diagnostics Market Scope
North America ovarian cancer diagnostics market is segmented into product type, procedure type, cancer type and end user. The growth amongst these segments will help you analyze meager growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
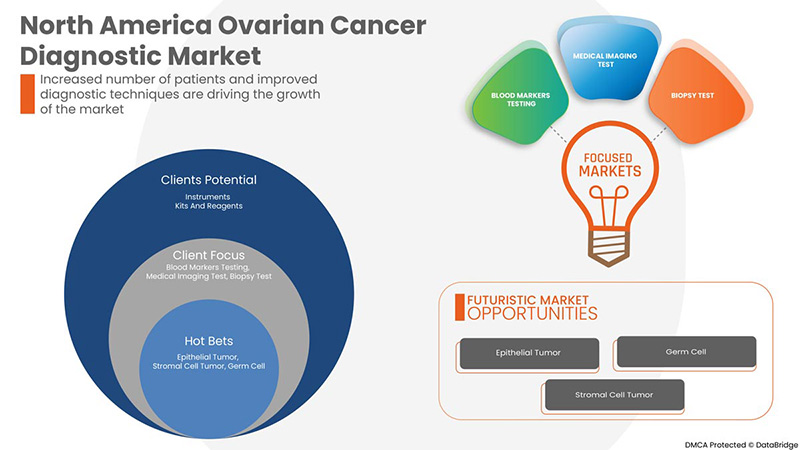
Product Type
- Instruments
- Kits and Reagents
On the basis of product type, the North America ovarian cancer diagnostics market is segmented into instruments and kits and reagents.
Procedure Type
- Blood Markers Testing
- Medical Imaging Test
- Biopsy Tests
- Genetic Testing
On the basis of procedure type, the North America ovarian cancer diagnostics market is segmented into blood markers testing, medical imaging test, biopsy tests and genetic testing
Cancer Type
- Epithelial Tumor
- Germ Cell
- Stromal Cell Tumor
On the basis of cancer type, the North America ovarian cancer diagnostics market is segmented into epithelial tumor, germ cell and stromal cell tumor
End User
- Cancer Diagnostic Centers
- Hospital Laboratories
- Research Institutes
- Others
On the basis of end user, the North America ovarian cancer diagnostics market is segmented into cancer diagnostic centers, hospital laboratories, research institutes and others
North America Ovarian Cancer Diagnostics Market Regional Analysis/Insights
The North America ovarian cancer diagnostics market is analyzed, and market size insights and trends are provided by country, product type, procedure type, cancer type and end user, as referenced above.
The countries covered in this market report U.S., Canada and Mexico.
U.S. is dominating the North America ovarian cancer diagnostics market in terms of market share and revenue and will continue to flourish its dominance during the forecast period. This is due to the high prevalence and incidence of neurological disorders in the region, and growing R&D investments and the launch of new products are boosting the market
The country section of the report also provides individual market impacting factors and changes in regulations in the market that impact the current and future trends of the market. Data points, such as new and replacement sales, country demographics, disease epidemiology, and import-export tariffs, are some of the major pointers used to forecast the market scenario for individual countries. In addition, the presence and availability of North American brands and their challenges faced due to competition from local and domestic brands and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and North America Ovarian Cancer Diagnostics Market Share Analysis
The ovarian cancer diagnostics market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, North America presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to Ovarian Cancer Diagnostics Market.
Some of the major players operating in the North America ovarian cancer diagnostics market are F. Hoffmann-la roche ltd, Tosoh india pvt. Ltd., Luminex corporation, Quest diagnostics incorporated, Thermo fisher scientific Inc., Ngenebio, Abbott, Siemens healthcare private limited, Myriad genetics Inc., Bio-rad laboratories, Inc., R&d systems, Inc., Foundation medicine, Inc., Abcam plc., Monobind Inc., Mp biomedicals, Biovision Inc., Boster biological technology, Biogenix Inc. Pvt. Ltd., Genway biotech and Lifespan biosciences, Inc among others.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET TESTING TYPE COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
4.3 GROWTH STRATEGIES ADOPTED BY KEY MARKET PLAYERS
5 INDUSTRY INSIGHTS
5.1 CONCLUSION
6 REGULATIONS OF THE NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 GROWING OVARIAN CANCER AWARENESS
7.1.2 IMPROVED DIAGNOSTIC PROCESSES AND TECHNIQUES
7.1.3 INCREASE IN NUMBER OF NEW CASES EVERY YEAR
7.1.4 IMPROVED IMAGING TECHNIQUES
7.2 RESTRAINS
7.2.1 HIGH COST OF DIAGNOSIS
7.2.2 ADVERSE EFFECTS OF THE TREATMENT
7.3 OPPORTUNITIES
7.3.1 INCREASING HEALTHCARE EXPENDITURE FOR CANCER DIAGNOSIS AND TREATMENT
7.3.2 GOVERNMENT INITIATIVES TOWARDS CANCER DIAGNOSTICS
7.4 CHALLENGES
7.4.1 STRICT REGULATIONS AND STANDARDS FOR THE APPROVAL AND COMMERCIALIZATION OF CANCER DIAGNOSTIC PRODUCTS
7.4.2 LACK OF SKILLED PROFESSIONALS
8 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE
8.1 OVERVIEW
8.2 INSTRUMENTS
8.3 KITS AND REAGENTS
9 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE
9.1 OVERVIEW
9.2 BLOOD MARKERS TESTING
9.3 MEDICAL IMAGING TEST
9.4 BIOPSY TEST
9.5 GENETIC TESTING
10 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE
10.1 OVERVIEW
10.2 EPITHELIAL TUMOR
10.3 GERM CELL
10.4 STROMAL CELL TUMOR
11 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER
11.1 OVERVIEW
11.2 CANCER DIAGNOSTIC CENTERS
11.3 HOSPITAL LABORATORIES
11.4 RESEARCH INSTITUTES
11.5 OTHERS
12 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY GEOGRAPHY
12.1 NORTH AMERICA
12.1.1 U.S.
12.1.2 CANADA
12.1.3 MEXICO
13 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
14 SWOT ANALYSIS
15 COMPANY PROFILE
15.1 F. HOFFMANN-LA ROCHE LTD
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENTS
15.2 TOSOH INDIA PVT. LTD.
15.2.1 COMPANY SNAPSHOT
15.2.2 COMPANY SHARE ANALYSIS
15.2.3 PRODUCT PORTFOLIO
15.2.4 RECENT DEVELOPMENT
15.3 LUMINEX CORPORATION (2022)
15.3.1 COMPANY SNAPSHOT
15.3.2 COMPANY SHARE ANALYSIS
15.3.3 PRODUCT PORTFOLIO
15.3.4 RECENT DEVELOPMENT
15.4 QUEST DIAGNOSTICS INCORPORATED (2022)
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENTS
15.5 THERMO FISHER SCIENTIFIC INC
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.5.5 RECENT DEVELOPMENT
15.6 ABBOTT
15.6.1 COMPANY SNAPSHOT
15.6.2 REVENUE ANALYSIS
15.6.3 PRODUCT PORTFOLIO
15.6.4 RECENT DEVELOPMENT
15.7 ABCAM PLC (2022)
15.7.1 COMPANY SNAPSHOT
15.7.2 REVENUE ANALYSIS
15.7.3 PRODUCT PORTFOLIO
15.7.4 1.7.4 RECENT DEVELOPMENT
15.8 BIOSUPPLY LTD
15.8.1 COMPANY SNAPSHOT
15.8.2 PRODUCT PORTFOLIO
15.8.3 RECENT DEVELOPMENT
15.9 BIO-RADBIO LABORATORIES
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENTS
15.1 BIOVISION INC.
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENT
15.11 BIOGENIX INC. PVT. LTD.
15.11.1 COMPANY SNAPSHOT
15.11.2 PRODUCT PORTFOLIO
15.11.3 RECENT DEVELOPMENT
15.12 BOSTER BIOLOGICAL TECHNOLOGY
15.12.1 COMPANY SNAPSHOT
15.12.2 PRODUCT PORTFOLIO
15.12.3 RECENT DEVELOPMENT
15.13 FOUNDATION MEDICINE
15.13.1 COMPANY SNAPSHOT
15.13.2 PRODUCT PORTFOLIO
15.13.3 RECENT DEVELOPMENT
15.14 FUJIREBIO
15.14.1 COMPANY SNAPSHOT
15.14.2 PRODUCT PORTFOLIO
15.14.3 RECENT DEVELOPMENT
15.15 GENWAY BIOTECH
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.16 INEX INNOVATIVE PRIVATE LIMITED
15.16.1 COMPANY SNAPSHOT
15.16.2 PRODUCT PORTFOLIO
15.16.3 RECENT DEVELOPMENT
15.17 LCM GENETIC SRL
15.17.1 COMPANY SNAPSHOT
15.17.2 PRODUCT PORTFOLIO
15.17.3 RECENT DEVELOPMENT
15.18 LIFESPAN BIOSCIENCES, INC
15.18.1 COMPANY SNAPSHOT
15.18.2 PRODUCT PORTFOLIO
15.18.3 RECENT DEVELOPMENTS
15.19 MP BIOMEDICALS
15.19.1 COMPANY SNAPSHOT
15.19.2 PRODUCT PORTFOLIO
15.19.3 RECENT DEVELOPMENT
15.2 MONOBIND INC.
15.20.1 COMPANY SNAPSHOT
15.20.2 PRODUCT PORTFOLIO
15.20.3 RECENT DEVELOPMENT
15.21 MYRIAD GENETICS, INC.
15.21.1 COMPANY SNAPSHOT
15.21.2 REVENUE ANALYSIS
15.21.3 PRODUCT PORTFOLIO
15.21.4 RECENT DEVELOPMENT
15.22 NGENEBIO
15.22.1 COMPANY SNAPSHOT
15.22.2 PRODUCT PORTFOLIO
15.22.3 RECENT DEVELOPMENTS
15.23 R&D SYSTEMS, INC.
15.23.1 COMPANY SNAPSHOT
15.23.2 PRODUCT PORTFOLIO
15.23.3 RECENT DEVELOPMENT
15.24 SIEMENS MEDICAL SOLUTIONS
15.24.1 COMPANY SNAPSHOT
15.24.2 REVENUE ANALYSIS
15.24.3 PRODUCT PORTFOLIO
15.24.4 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
List of Table
TABLE 1 24-MONTH EPISODE-OF-CARE COSTS FOR EARLY-STAGE AND LATE-STAGE CANCERS BY PAYER (USD BILLION)
TABLE 2 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 3 NORTH AMERICA INSTRUMENTS IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA KITS AND REAGENTS IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA BLOOD MARKERS TESTING IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA MEDICAL IMAGING TEST IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA BIOPSY TEST IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA GENETIC TESTING IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA EPITHELIAL TUMOR IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA GERM CELL IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA STROMAL CELL TUMOR IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA CANCER DIAGNOSTIC CENTERS IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA HOSPITAL LABORATORIES IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA RESEARCH INSTITUTES IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA OTHERS IN OVARIAN CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 24 U.S. OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 25 U.S. OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 26 U.S. OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 27 U.S. OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 28 CANADA OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 29 CANADA OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 30 CANADA OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 31 CANADA OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 32 MEXICO OVARIAN CANCER DIAGNOSTICS MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 33 MEXICO OVARIAN CANCER DIAGNOSTICS MARKET, BY PROCEDURE TYPE, 2021-2030 (USD MILLION)
TABLE 34 MEXICO OVARIAN CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 35 MEXICO OVARIAN CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
List of Figure
FIGURE 1 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET : SEGMENTATION
FIGURE 2 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET : DATA TRIANGULATION
FIGURE 3 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET : DROC ANALYSIS
FIGURE 4 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET : NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET : COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET : INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET : DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET : MARKET TESTING TYPE COVERAGE GRID
FIGURE 9 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET : VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET : SEGMENTATION
FIGURE 11 THE INCREASE IN THE AWARENESS ABOUT OVARIAN CANCER IS EXPECTED TO DRIVE THE NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET IN THE FORECAST PERIOD OF 2022 TO 2030
FIGURE 12 PRODUCT SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET IN 2022 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGE OF THE NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET
FIGURE 14 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, 2022
FIGURE 15 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, 2023-2030 (USD MILLION)
FIGURE 16 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, CAGR (2023-2030)
FIGURE 17 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 18 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY PROCEDURE TYPE, 2022
FIGURE 19 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
FIGURE 20 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY PROCEDURE TYPE, CAGR (2023-2030)
FIGURE 21 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY PROCEDURE TYPE, LIFELINE CURVE
FIGURE 22 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, 2022
FIGURE 23 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, 2023-2030 (USD MILLION)
FIGURE 24 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, CAGR (2023-2030)
FIGURE 25 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, LIFELINE CURVE
FIGURE 26 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY END USER, 2022
FIGURE 27 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 28 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 29 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY END USER, LIFELINE CURVE
FIGURE 30 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 31 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 32 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 33 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 34 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: BY PRODUCT TYPE (2023-2030)
FIGURE 35 NORTH AMERICA OVARIAN CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












