North America Persistent Corneal Epithelial Defects Treatment Market Analysis and Size
North America persistent corneal epithelial defects treatment market is expected to witness significant growth during the forecast period. Although numerous therapies exist and a growing number of novel approaches are evolving, treatment of this disease can still be quite challenging. It is necessary to treat the underlying causative condition, which includes infection, limbal stem cell deficiency, or diabetes, to facilitate wound healing. The introduction of the latest technology development which offers various therapies for treating various complications caused by the disorder and the rise in incidence of dry eye syndrome boost market growth.
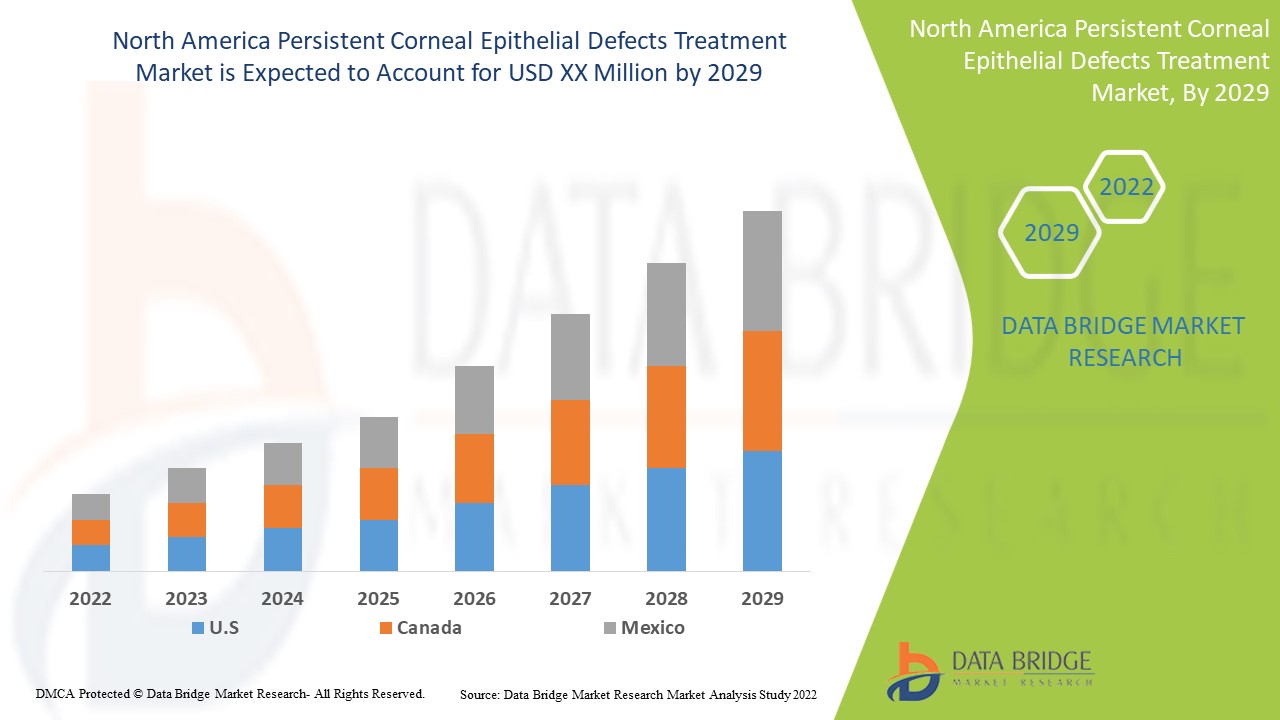
Data Bridge Market Research analyses a growth rate in the North America persistent corneal epithelial defects treatment market in the forecast period 2022-2029. The market was valued at USD 98.8 million in 2021. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
North America Persistent Corneal Epithelial Defects Treatment Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2014 - 2019) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Clinical Causes (Inflammatory Disease, Neurotrophic Keratitis (NK), Epithelial/Limbal Stem Cell Deficiency, Others), Type (Devices, Medication), End User (Hospital/Clinical Laboratories, Physician Offices, Reference Laboratories and Other End Users), Distribution Channel (Direct Tender, Retail Sales and Other) |
|
Countries Covered |
U.S., Canada, Mexico |
|
Market Players Covered |
Dompé farmaceutici S.p.A (Italy), Novartis AG (Switzerland), Abbvie,Inc (U.S.), Johnson & Johnson Services, Inc. (India), Laboratoires Théa. (France), Almirall, S.A (Spain), Ocular Science (U.S.), Kala Pharmaceuticals (U.S.), Bausch Health Companies Inc (Canada), Integra LifeSciences (U.S.), BioTissue (Florida) |
|
Market Opportunities |
|
Market Definition
Persistent Corneal Epithelial Defects (PCED/ PED) are mainly characterized by the failure of rapid re-epithelialization and closure within the range of 10-14 days after a corneal injury, combined with standard supportive treatment. This corneal condition results in the disturbance of the corneal surface and the exposed eyes are vulnerable to stromal ulceration, scarring, and infections.
Persistent Corneal Epithelial Defects Treatment Market Dynamics
Drivers
- Increased Partnerships and Collaborations
The rising partnerships and collaborations between several companies boost the market's growth. For instance, in October 2017, Bio-Tissue entered a strategic agreement with Optima Pharmazeutische GmbH, a pharmaceutical company that offers otorhinolaryngology, ophthalmology, and pneumology products. This agreement helps the company to distribute its Natural Ocular Cleanser in Germany. Thus, all these collaborations boost the growth of the market.
- Higher Demand of Hospitals
The rising demand of hospitals increase the market growth rapidly. Most chronic disease diagnostics are majorly carried out in hospitals as they are very complex and it requires technologically advanced products and thus this is driving the market for hospital/clinical laboratories.
Opportunities
- Rise in Eye Surgeries and Contact Lenses
Increasing cases of eye surgeries and the higher usage of contact lenses is anticipated to boost the market growth. For instance, in 2019, Cindy Tromans published a study on “Therapeutic Contact Lenses”. The study expected that a persistent epithelial defect (PED) can have numerous different etiologies that includes viral or fungal corneal, bacterial infections PED can occur after thermal or chemical burns that leads to slow healing after surgery. Development of novel therapeutics will boost the market and create new opportunities for the growth.
- Increasing Demand of Treatment Types
Lubricant eye drops are the first line treatment for the PCED treatment, but are generally insufficient to treat PCED. Oxervate is one of the most effective treatment methods for persistent corneal epithelial defects treatment was launched in January 2019, so the acceptability is very low for this product. and the pharmaceutical product are more costly compared to device such as Oxervate costs USD 47,200, whereas punctal plug costs approximately USD 27 and bandage contact lens cost about USD 57. Thus, increasing demand for the treatmnet types boost the market's growth.
Restraints/Challenges
- Lack of skilled professionals
The lack of qualified personnel who cannot treat the patients with appropriate treatments could curb the growth of the North America persistent corneal epithelial defects treatment market over a forecast period.
- High Cost
The huge expenditure required for setting up these techniques hamper the market growth. Several market players make huge investment in installing new and advanced machines to faster the process and in return the cost is increased.
This North America persistent corneal epithelial defects treatment market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the North America persistent corneal epithelial defects treatment market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
North America Persistent Corneal Epithelial Defects Treatment Market
The North America persistent corneal epithelial defects treatment market is segmented on the basis of clinical causes, type, end-user and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Clinical Causes
- Inflammatory Disease
- Neurotrophic Keratitis (NK)
- Epithelial/Limbal Stem Cell Deficiency
- Others
Type
- Devices
- Medication
End User
- Hospital/Clinical Laboratories
- Physician Offices
- Reference Laboratories
- Others
Distribution Channel
- Direct Tender
- Retail Sales
- Other
North America Persistent Corneal Epithelial Defects Treatment Market Regional Analysis/Insights
The North America persistent corneal epithelial defects treatment market is analyzed and market size insights and trends are provided by clinical causes, type, end-user and distribution channel as referenced above.
The major countries covered in the North America persistent corneal epithelial defects treatment market report are the U.S., Canada, Mexico.
North America persistent corneal epithelial defects treatment market is growing due to increase in mechanical traumas cases at the significant growth rate in the forecast period of 2022 to 2029.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and North America Persistent Corneal Epithelial Defects Treatment Market Share Analysis
The North America persistent corneal epithelial defects treatment market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to North America persistent corneal epithelial defects treatment market
Key players operating in the North America persistent corneal epithelial defects treatment market t include:
- Dompé farmaceutici S.p.A (Italy)
- Novartis AG (Switzerland)
- Abbvie,Inc (U.S.)
- Johnson & Johnson Services, Inc. (India)
- Laboratoires Théa. (France)
- Almirall, S.A (Spain)
- Ocular Science (U.S.)
- Kala Pharmaceuticals (U.S.)
- Bausch Health Companies Inc (Canada)
- Integra LifeSciences (U.S.)
- BioTissue (Florida)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












