全球實體器官移植市場,依器官(腎臟、肝臟、胰臟、心臟、肺、小腸、腎臟/胰臟等)、產品(組織產品、免疫抑制藥物、保存液)、治療(免疫抑制劑、單株抗體等)、最終使用者(醫院、居家照護、專科中心等)、分銷通路(醫院藥局、網路藥局、零售藥局(醫院、家庭護理、專科中心等)、分銷通路(醫院藥局、網路藥局、零售藥局)-209 年 209 年。
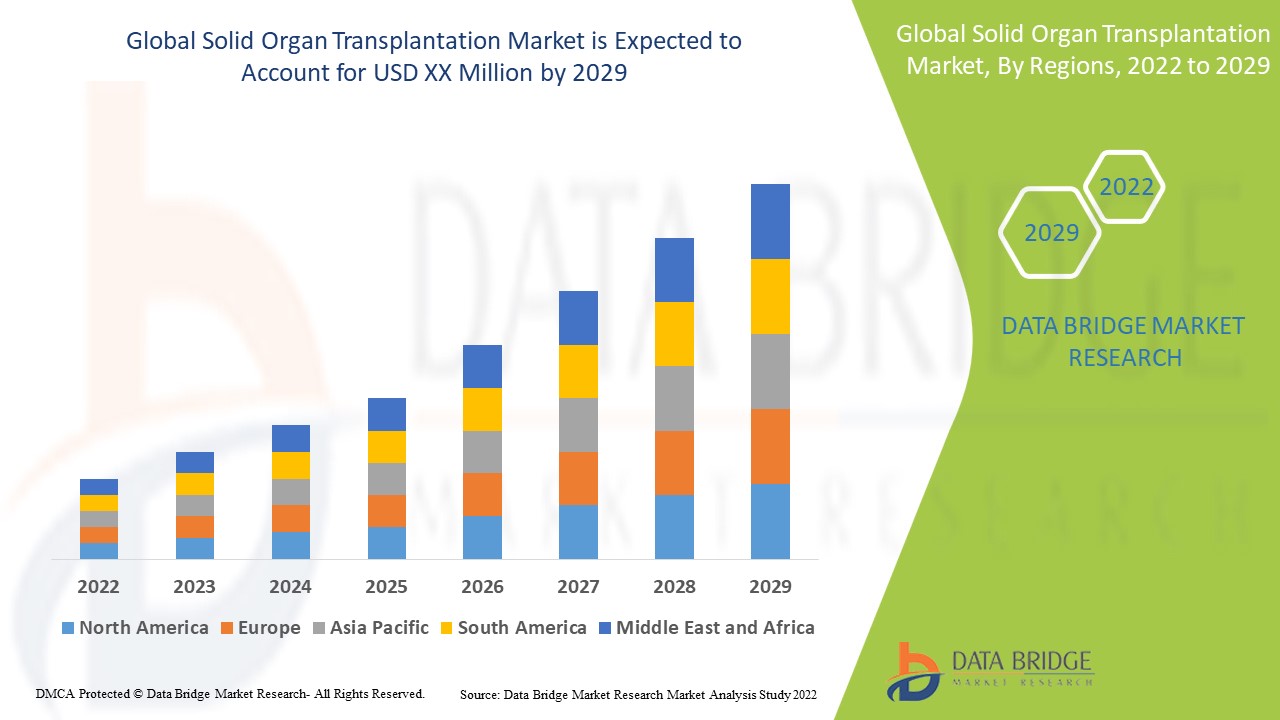
實體器官移植市場分析及規模
預計全球實體器官移植市場在預測期內將顯著成長。對新型組織移植產品和用於治療器官衰竭的器官移植的需求不斷增長是推動市場成長的主要因素。不健康的飲食習慣、缺乏運動、飲酒和藥物濫用是導致器官衰竭的一些重要原因。據觀察,組織產品是2021年創收最高的細分市場,市佔率為57.8%。新冠疫情也對市場成長產生了重大影響。
數據橋市場研究公司 (Data Bridge Market Research) 分析了 2022-2029 年預測期間全球實體器官移植市場的成長率。除了市場價值、成長率、細分市場、地理覆蓋範圍和主要參與者等市場情境洞察外,數據橋市場研究公司整理的市場報告還包含深度專家分析、病患流行病學、產品線分析、定價分析和監管框架。
市場定義
實體器官移植對於末期腎臟、胰臟、小腸、心臟、肝臟和肺部疾病患者來說是一個優勢。雖然實體器官移植並不能提高存活率,但血管複合異體移植和子宮移植可以一定程度上改善患者的生活品質。實體器官移植對醫療保健產業至關重要,因此預計在預測期內將大幅成長。
實體器官移植市場範圍和細分
|
報告指標 |
細節 |
|
預測期 |
2022年至2029年 |
|
基準年 |
2021 |
|
歷史性的歲月 |
2020(可自訂至 2014 - 2019) |
|
定量單位 |
收入(百萬美元)、銷售(單位)、定價(美元) |
|
涵蓋的領域 |
器官(腎臟、肝臟、胰臟、心臟、肺、小腸、腎臟/胰臟、其他)、產品(組織產品、免疫抑制藥物、保存液)、治療(免疫抑制劑、單株抗體、其他)、最終使用者(醫院、家庭護理、專科中心、其他)、分銷管道(醫院藥房、網路藥房、零售藥房) |
|
覆蓋國家 |
北美洲的美國、加拿大和墨西哥、德國、法國、英國、荷蘭、瑞士、比利時、俄羅斯、義大利、西班牙、土耳其、歐洲的其他歐洲國家、中國、日本、印度、韓國、新加坡、馬來西亞、澳洲、泰國、印尼、菲律賓、亞太地區 (APAC) 的其他亞太國家 (APAC)、沙烏地阿拉伯、阿拉伯聯合大公國、南非、埃及、以色列、中東和其他國家的非洲國家 (MEA) |
|
涵蓋的市場參與者 |
Teva Pharmaceutical Industries Ltd(以色列)、Mylan NV(美國)、Johnsons & Johnsons Services Inc(美國)、F. Hoffman-La Roche Ltd.(瑞士)、Lilly(美國)、Merck & Co., Inc.(美國)、Aurobindo Pharma(印度)、Bristol-Myers Squit Company(美國)、丹麥)、KGSA. A/S(丹麥)、Genentech, Inc(美國)、Celltrion Inc(韓國) |
|
市場機會 |
|
全球實體器官移植市場動態
驅動程式
- 器官移植需求不斷成長
全球器官移植需求的成長可歸因於急性疾病發生率的上升,這反過來又導致器官衰竭數量增加。例如,糖尿病和高血壓被認為是末期腎病最常見的原因,而腎臟移植或透析是維持患者生命的唯一治療選擇。根據美國衛生與公眾服務部的記錄,2019年美國約有122,913名患者正在等待器官移植。因此,用於治療器官衰竭的先進移植產品的需求很高,這推動了市場的成長。
- 技術進步
該領域的多項技術進步預計將推動市場成長。例如,2015年9月,Arthrex公司推出了ArthroFlex去細胞真皮基質,這是一款擬用於關節囊重建的新型骨生物學產品。該產品能夠保留其生長因子、天然膠原支架和彈性蛋白,並允許移植材料成功植入受體體內。所有這些因素都推動了市場成長。
機會
- 更高的併購
一些製造商正致力於新產品開發、併購和合作,以擴大現有產品組合併保持其在市場上的持續地位。例如,2016年9月,史賽克公司宣布收購Instratek。 Instratek是一家領先的踝關節、足部和上肢手術微創軟組織萎縮器材製造商。
- 免疫抑制藥物銷售成長
2021年,用於移植的組織產品佔據了最大的市場。預計未來這一類別仍將保持其主導地位,因為骨科手術中越來越多地使用骨科生物製劑來恢復正常功能、促進組織癒合和減輕疼痛,並增加全球老齡人口的不斷增長。此外,該領域的新創公司正在獲得投資以拓展業務。因此,這些因素為市場成長創造了巨大的機會。
限制/挑戰
- 高成本
與器官移植和許多其他免疫抑制藥物的巨大關聯抑制了市場的成長。
- 無法獲得治療
並非所有國家都能提供所有治療方法,這限制了市場的成長。在一些欠發達國家,改良的治療方法尚未普及。因此,這限制了市場的成長。
這份全球實體器官移植市場報告詳細介紹了最新發展動態、貿易法規、進出口分析、生產分析、價值鏈優化、市場份額、國內和本地市場參與者的影響,並分析了新興收入來源、市場法規變化、戰略市場增長分析、市場規模、類別市場增長、應用領域和主導地位、產品審批、產品發布、地擴展以及市場技術創新等方面的機遇。如需了解更多關於全球實體器官移植市場的信息,請聯繫 Data Bridge 市場研究公司獲取分析師簡報,我們的團隊將幫助您做出明智的市場決策,實現市場成長。
COVID-19對全球實體器官移植市場的影響
由於有相當一部分新冠病毒患者在醫療中心住院,管理機構不得不對這些患者進行限制。疫情也導致全球器官移植手術登記人數下降。
移植和器官捐贈手術數量的減少主要是由於外科醫生工作繁忙、資源匱乏、封鎖和旅行限制等因素。由於存在感染的可能性,COVID-19 檢測呈陽性的患者被限制捐贈器官。此外,促使移植手術的另一個主要擔憂是免疫抑制劑可能與 COVID-19 治療藥物相互作用,這已導致大量移植捐贈者出現器官衰竭。
近期發展:
- 2020年11月,魯賓在美國市場推出了免疫抑制劑仿製藥他克莫司,用於預防同種異體肝臟、腎臟和心臟移植的器官排斥。
全球實體器官移植市場範圍
全球實體器官移植市場根據器官、產品、治療、分銷管道和最終用戶進行細分。這些細分市場的成長將有助於您分析行業中成長乏力的細分領域,並為用戶提供有價值的市場概覽和市場洞察,幫助他們做出策略決策,確定核心市場應用。
器官
- 腎
- 肝
- 胰臟
- 心
- 肺
- 小腸
- 腎臟/胰腺
- 其他的
產品
- 紙巾產品
- 免疫抑制藥物
- 保存液
治療
- 免疫抑制
- 單株抗體
- 其他的
最終用戶
- 醫院
- 居家護理
- 專業中心
- 其他的
分銷管道
- 醫院藥房
- 網路藥局
- 零售藥局
實體器官移植市場區域分析/洞察
對全球實體器官移植市場進行了分析,並按上述器官、產品、治療、分銷管道和最終用戶提供了市場規模洞察和趨勢。
全球實體器官移植市場報告涵蓋的主要國家 有:北美洲的美國、加拿大和墨西哥、歐洲的德國、法國、英國、荷蘭、瑞士、比利時、俄羅斯、義大利、西班牙、土耳其、歐洲其他地區、亞太地區(APAC)的中國、日本、印度、韓國、新加坡、馬來西亞、澳洲、泰國、印尼、菲律賓、亞太地區(APAC)的其他地區、沙烏地阿拉伯、阿聯酋、南非、埃及、以色列、中東和非洲(MEA)的其他地區、南美洲的巴西、阿根廷和南美洲其他地區。
由於該產品的關鍵製造以及醫療保健和研發支出的增加,預計北美將擁有最高的市場成長。
由於器官衰竭盛行率增加以及仿製藥製造商的存在,亞太地區佔據了市場主導地位。
報告的國家部分還提供了各個市場的影響因素以及國內市場監管變化,這些變化將影響市場的當前和未來趨勢。此外,在對國家/地區數據進行預測分析時,還考慮了全球品牌的存在和可用性,以及它們因本土和國內品牌的激烈或稀缺競爭而面臨的挑戰,以及國內關稅和貿易路線的影響。
競爭格局與全球實體器官移植市場份額分析
全球實體器官移植市場競爭格局提供了按競爭對手劃分的詳細資訊。詳細資訊包括公司概況、公司財務狀況、收入、市場潛力、研發投入、新市場舉措、全球佈局、生產基地和設施、生產能力、公司優勢和劣勢、產品發布、產品寬度和廣度以及應用主導地位。以上提供的數據僅與公司在全球實體器官移植市場的重點相關。
全球實體器官移植市場的主要參與者包括:
- 梯瓦製藥工業股份有限公司(以色列)
- Mylan NV(美國)
- 強生服務公司(美國)
- F. Hoffman-La Roche Ltd.(瑞士)
- 禮來(美國)
- 默克公司(美國)
- 奧羅賓多製藥(印度)
- 百時美施貴寶公司(美國)
- 葛蘭素史克公司(英國)
- Ascendis Pharma A/S(丹麥)
- LEO Pharma A/S(丹麥)
- 基因泰克公司(美國)
- Celltrion Inc(韓國)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL SOLID ORGAN TRANSPLANTATION MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL SOLID ORGAN TRANSPLANTATION MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY MODELING
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL SOLID ORGAN TRANSPLANTATION MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 PATENT ANALYSIS
6.1.1 PATENT LANDSCAPE
6.1.2 USPTO NUMBER
6.1.3 PATENT EXPIRY
6.1.4 EPIO NUMBER
6.1.5 PATENT STRENGTH AND QUALITY
6.1.6 PATENT CLAIMS
6.1.7 PATENT CITATIONS
6.1.8 PATENT LITIGATION AND LICENSING
6.1.9 FILE OF PATENT
6.1.10 PATENT RECEIVED CONTRIES
6.1.11 TECHNOLOGY BACKGROUND
6.2 DRUG TREATMENT RATE BY MATURED MARKETS
6.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.4 PATIENT FLOW DIAGRAM
6.5 KEY PRICING STRATEGIES
6.6 KEY PATIENT ENROLLMENT STRATEGIES
6.7 INTERVIEWS WITH SPECIALIST
6.8 OTHER KOL SNAPSHOTS
7 EPIDEMIOLOGY
7.1 INCIDENCE OF ALL BY GENDER
7.2 TREATMENT RATE
7.3 MORTALITY RATE
7.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
7.5 PATIENT TREATMENT SUCCESS RATES
8 MERGERS AND ACQUISITION
8.1 LICENSING
8.2 COMMERCIALIZATION AGREEMENTS
9 REGULATORY FRAMEWORK
9.1 REGULATORY APPROVAL PROCESS
9.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
9.3 REGULATORY APPROVAL PATHWAYS
9.4 LICENSING AND REGISTRATION
9.5 POST-MARKETING SURVEILLANCE
9.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
10 PIPELINE ANALYSIS
10.1 CLINICAL TRIALS AND PHASE ANALYSIS
10.2 DRUG THERAPY PIPELINE
10.3 PHASE III CANDIDATES
10.4 PHASE II CANDIDATES
10.5 PHASE I CANDIDATES
10.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 GLOBAL CLINICAL TRIAL MARKET FOR SOLID ORGAN TRANSPLANTATION MARKET
Company Name Therapeutic Area
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE SOLID ORGAN TRANSPLANTATION MARKET
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved But Not Yet Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE SOLID ORGAN TRANSPLANTATION MARKET
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE SOLID ORGAN TRANSPLANTATION MARKET
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR SOLID ORGAN TRANSPLANTATION MARKET
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
11 MARKETED DRUG ANALYSIS
11.1 DRUG
11.1.1 BRAND NAME
11.1.2 GENERICS NAME
11.2 THERAPEUTIC INDICTION
11.3 PHARMACOLOGICAL CLASS OF THE DRUG
11.4 DRUG PRIMARY INDICATION
11.5 MARKET STATUS
11.6 MEDICATION TYPE
11.7 DRUG DOSAGES FORM
11.8 DOSAGES AVAILABILITY
11.9 DRUG ROUTE OF ADMINISTRATION
11.1 DOSING FREQUENCY
11.11 DRUG INSIGHT
11.12 AN OVERVIEW OF THE DRUG DEVELOPMENT ACTIVITIES SUCH AS REGULATORY MILSTONE, SAFETY DATA AND EFFICACY DATA, MARKET EXCLUSIVITY DATA.
11.12.1 FORECAST MARKET OUTLOOK
11.12.2 CROSS COMPETITION
11.12.3 THERAPEUTIC PORTFOLIO
11.12.4 CURRENT DEVELOPMENT SCENARIO
12 MARKET ACCESS
12.1 10-YEAR MARKET FORECAST
12.2 CLINICAL TRIAL RECENT UPDATES
12.3 ANNUAL NEW FDA APPROVED DRUGS
12.4 DRUGS MANUFACTURER AND DEALS
12.5 MAJOR DRUG UPTAKE
12.6 CURRENT TREATMENT PRACTICES
12.7 IMPACT OF UPCOMING THERAPY
13 R & D ANALYSIS
13.1 COMPARATIVE ANALYSIS
13.2 DRUG DEVELOPMENTAL LANDSCAPE
13.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
13.4 THERAPEUTIC ASSESSMENT
13.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
14 MARKET OVERVIEW
14.1 DRIVERS
14.2 RESTRAINTS
14.3 OPPORTUNITIES
14.4 CHALLENGES
15 GLOBAL SOLID ORGAN TRANSPLANTATION MARKET, BY ORGAN
15.1 OVERVIEW
15.2 KIDNEY TRANSPLANTATION
15.3 LIVER TRANSPLANTATION
15.4 HEART TRANSPLANTATION
15.5 LUNG TRANSPLANTATION
15.6 PANCREAS TRANSPLANTATION
15.7 SMALL BOWEL TRANSPLANTATION
15.8 OTHERS
16 GLOBAL SOLID ORGAN TRANSPLANTATION MARKET, BY PRODUCT
16.1 OVERVIEW
16.2 THERAPY
16.2.1 IMMUNOSUPPRESSIVE DRUGS
16.2.1.1. CALCINEURIN INHIBITORS (CNI)
16.2.1.1.1. CYCLOSPORINE
16.2.1.1.1.1 NEORAL
16.2.1.1.1.2 SANDIMMUNE
16.2.1.1.1.3 OTHERS
16.2.1.1.2. TACROLIMUS (PROGRAF)
16.2.1.1.3. OTHERS
16.2.1.2. ANTIPROLIFERATIVE AGENTS
16.2.1.2.1. AZATHIOPRINE (IMURAN)
16.2.1.2.2. MYCOPHENOLATE MOFETIL (MMF)
16.2.1.2.3. CYCLOPHOSPHAMIDE
16.2.1.2.4. LEFLUNOMIDE
16.2.1.2.5. OTHERS
16.2.1.3. MTOR INHIBITORS
16.2.1.3.1. SIROLIMUS
16.2.1.3.2. EVEROLIMUS
16.2.1.3.3. TEMSIROLIMUS
16.2.1.3.4. RIDAFOROLIMUS
16.2.1.3.5. OTHERS
16.2.1.4. CORTICOSTEROIDS
16.2.1.4.1. METHYLPREDNISOLONE
16.2.1.4.2. DEXAMETHASONE
16.2.1.4.3. PREDNISOLONE
16.2.1.4.4. OTHERS
16.2.1.5. STATIN THERAPY
16.2.1.6. ALKYLATING AGENT
16.2.1.7. INTERLEUKIN INHIBITORS
16.2.1.8. SPECIFIC LYMPHOCYTE-SIGNALING INHIBITORS
16.2.2 ANTI-DRUG ANTIBODIES
16.2.2.1. MONOCLONAL ANTIBODIES
16.2.2.1.1. MARKETED
16.2.2.1.1.1 MUROMONAB-CD3
16.2.2.1.1.2 BASILIXIMAB
16.2.2.1.1.3 BELATACEPT
16.2.2.1.1.4 ALEMTUZUMAB
16.2.2.1.1.5 OTHERS
16.2.2.1.2. INVESTIGATIONAL
16.2.2.1.2.1 ALEMTUZUMAB
16.2.2.1.2.2 RITUXIMAB
16.2.2.1.2.3 OTHERS
16.2.2.2. TNF-ALPHA INHIBITORS
16.2.2.2.1. INFLIXIMAB
16.2.2.2.2. ADALIMUMAB
16.2.2.2.3. OTHERS
16.2.2.3. OTHERS
16.2.3 THERAPEUTIC PLASMA EXCHANGE (TPE)
16.2.4 INTRAVENOUS IMMUNOGLOBULIN (IVIG)
16.3 PRESERVATION SOLUTION
16.3.1 BY SOLUTION TYPE
16.3.1.1. UW
16.3.1.2. EURO-COLLINS
16.3.1.3. BELZER'S MPS
16.3.1.4. CUSTODIOL HTK
16.3.1.5. CELSIOR
16.3.1.6. PERFADEX
16.3.1.7. POLYSOL
16.3.1.8. VIASPAN
16.3.1.9. OTHERS
16.3.2 BY METHOD OF PRESERVATION
16.3.2.1. STATIC PRESERVATION METHOD
16.3.2.2. DYNAMIC PRESERVATION METHOD
16.3.2.2.1. HYPOTHERMIC MACHINE PERFUSION PERSERVATION
16.3.2.2.2. NORMOTHERIC MACHINE PERFUSION
16.3.2.2.3. OXYGEN PERSUFFLATION
16.3.2.2.4. OTHERS
16.3.2.3. OTHERS
16.4 TISSUE PRODUCTS
16.5 OTHERS
17 GLOBAL SOLID ORGAN TRANSPLANTATION MARKET, BY ROUTE OF ADMINISTRATION
17.1 ORAL
17.1.1 TABLET
17.1.2 CAPSULE
17.1.3 OTHERS
17.2 PARENTRAL
17.2.1 INTRAVENEOUS
17.2.2 SUBCUTANEOUS
17.2.3 OTHERS
17.3 OTHERS
18 GLOBAL SOLID ORGAN TRANSPLANTATION MARKET, BY TYPE OF ORGAN DONATION
18.1 OVERVIEW
18.2 DECEASED DONOR
18.3 LIVING DONOR
19 GLOBAL SOLID ORGAN TRANSPLANTATION MARKET, BY DRUG BRAND
19.1 OVERVIEW
19.2 BRANDED
19.2.1 PROGRAF
19.2.2 CELLCEPT
19.2.3 ZORTRESS
19.2.4 RAPAMUNE
19.2.5 OTHERS
19.3 GENERICS
20 GLOBAL SOLID ORGAN TRANSPLANTATION MARKET, BY PRESCRIPTION MODE
20.1 OVERVIEW
20.2 OVER THE COUNTER (OTC)
20.3 PRESCRIPTION BASED
21 GLOBAL SOLID ORGAN TRANSPLANTATION MARKET, BY POPULATION TYPE
21.1 OVERVIEW
21.2 MALE
21.2.1 YOUNG (BELOW 30 YEARS)
21.2.2 MIDDLE AGE (30–50 YEARS)
21.2.3 ELDERLY (65 YEARS OR OLDER)
21.3 FEMALE
21.3.1 YOUNG (BELOW 30 YEARS)
21.3.2 MIDDLE AGE (30–50 YEARS)
21.3.3 ELDERLY (65 YEARS OR OLDER)
22 GLOBAL SOLID ORGAN TRANSPLANTATION MARKET, BY END USER
22.1 OVERVIEW
22.2 HOSPITAL
22.2.1 BY TYPE
22.2.1.1. PUBLIC
22.2.1.2. PRIVATE
22.2.2 BY TIER
22.2.2.1. TIER 1
22.2.2.2. TIER 2
22.2.2.3. TIER 3
22.3 SPECIALTY CLINICS
22.4 HOME HEALTHCARE
22.5 AMBULATORY SURGICAL CENTERS
22.6 ACADEMIC AND RESEARCH INSTITUTES
22.7 OTHERS
23 GLOBAL SOLID ORGAN TRANSPLANTATION MARKET, BY DISTRIBUTION CHANNEL
23.1 OVERVIEW
23.2 DIRECT TENDER
23.3 REATIL SALES
23.3.1 OFFLINE PHARMACY
23.3.1.1. HOSPITAL PHARMACY
23.3.1.2. MEDICAL STORES AND DRUGS
23.3.1.3. OTHERS
23.3.2 ONLINE PHARMACY
23.3.2.1. E-STORES
23.3.2.2. COMPANY WEBSITE
23.3.2.3. OTHERS
23.4 OTHERS
24 GLOBAL SOLID ORGAN TRANSPLANTATION MARKET, COMPANY LANDSCAPE
24.1 COMPANY SHARE ANALYSIS: GLOBAL
24.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
24.3 COMPANY SHARE ANALYSIS: EUROPE
24.4 COMPANY SHARE ANALYSIS: ASIA PACIFIC
24.5 COMPANY SHARE ANALYSIS: MIDDLE EAST AND AFRICA
24.6 MERGERS & ACQUISITIONS
24.7 NEW PRODUCT DEVELOPMENT & APPROVALS
24.8 EXPANSIONS
24.9 REGULATORY CHANGES
24.1 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
25 GLOBAL SOLID ORGAN TRANSPLANTATION MARKET, BY GEOGRAPHY
GLOBAL SOLID ORGAN TRANSPLANTATION MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
25.1 NORTH AMERICA
25.1.1 U.S.
25.1.2 CANADA
25.1.3 MEXICO
25.2 EUROPE
25.2.1 GERMANY
25.2.2 FRANCE
25.2.3 U.K.
25.2.4 HUNGARY
25.2.5 LITHUANIA
25.2.6 AUSTRIA
25.2.7 IRELAND
25.2.8 NORWAY
25.2.9 POLAND
25.2.10 ITALY
25.2.11 SPAIN
25.2.12 RUSSIA
25.2.13 TURKEY
25.2.14 NETHERLANDS
25.2.15 SWITZERLAND
25.2.16 REST OF EUROPE
25.3 ASIA-PACIFIC
25.3.1 JAPAN
25.3.2 CHINA
25.3.3 SOUTH KOREA
25.3.4 INDIA
25.3.5 AUSTRALIA
25.3.6 SINGAPORE
25.3.7 THAILAND
25.3.8 MALAYSIA
25.3.9 INDONESIA
25.3.10 PHILIPPINES
25.3.11 VIETNAM
25.3.12 REST OF ASIA-PACIFIC
25.4 SOUTH AMERICA
25.4.1 BRAZIL
25.4.2 ARGENTINA
25.4.3 PERU
25.4.4 REST OF SOUTH AMERICA
25.5 MIDDLE EAST AND AFRICA
25.5.1 SOUTH AFRICA
25.5.2 GLOBAL
25.5.3 UAE
25.5.4 EGYPT
25.5.5 KUWAIT
25.5.6 ISRAEL
25.5.7 REST OF MIDDLE EAST AND AFRICA
25.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
26 GLOBAL SOLID ORGAN TRANSPLANTATION MARKET, SWOT AND DBMR ANALYSIS
27 GLOBAL SOLID ORGAN TRANSPLANTATION MARKET, COMPANY PROFILE
27.1 TEVA PHARMACEUTICALS USA, INC.
27.1.1 COMPANY OVERVIEW
27.1.2 REVENUE ANALYSIS
27.1.3 GEOGRAPHIC PRESENCE
27.1.4 PRODUCT PORTFOLIO
27.1.5 RECENT DEVELOPMENTS
27.2 WEEFSELPHARMA.COM
27.2.1 COMPANY OVERVIEW
27.2.2 REVENUE ANALYSIS
27.2.3 GEOGRAPHIC PRESENCE
27.2.4 PRODUCT PORTFOLIO
27.2.5 RECENT DEVELOPMENTS
27.3 ADVACARE PHARMA
27.3.1 COMPANY OVERVIEW
27.3.2 REVENUE ANALYSIS
27.3.3 GEOGRAPHIC PRESENCE
27.3.4 PRODUCT PORTFOLIO
27.3.5 RECENT DEVELOPMENTS
27.4 NOVARTIS AG
27.4.1 COMPANY OVERVIEW
27.4.2 REVENUE ANALYSIS
27.4.3 GEOGRAPHIC PRESENCE
27.4.4 PRODUCT PORTFOLIO
27.4.5 RECENT DEVELOPMENTS
27.5 DR. REDDY’S LABORATORIES, INC.
27.5.1 COMPANY OVERVIEW
27.5.2 REVENUE ANALYSIS
27.5.3 GEOGRAPHIC PRESENCE
27.5.4 PRODUCT PORTFOLIO
27.5.5 RECENT DEVELOPMENTS
27.6 APOTEX INC.
27.6.1 COMPANY OVERVIEW
27.6.2 REVENUE ANALYSIS
27.6.3 GEOGRAPHIC PRESENCE
27.6.4 PRODUCT PORTFOLIO
27.6.5 RECENT DEVELOPMENTS
27.7 STRIDES PHARMA SCIENCE LIMITED
27.7.1 COMPANY OVERVIEW
27.7.2 REVENUE ANALYSIS
27.7.3 GEOGRAPHIC PRESENCE
27.7.4 PRODUCT PORTFOLIO
27.7.5 RECENT DEVELOPMENTS
27.8 ASTELLAS PHARMA US, INC.
27.8.1 COMPANY OVERVIEW
27.8.2 REVENUE ANALYSIS
27.8.3 GEOGRAPHIC PRESENCE
27.8.4 PRODUCT PORTFOLIO
27.8.5 RECENT DEVELOPMENTS
27.9 ACCORD HEALTHCARE US
27.9.1 COMPANY OVERVIEW
27.9.2 REVENUE ANALYSIS
27.9.3 GEOGRAPHIC PRESENCE
27.9.4 PRODUCT PORTFOLIO
27.9.5 RECENT DEVELOPMENTS
27.1 BIOCON
27.10.1 COMPANY OVERVIEW
27.10.2 REVENUE ANALYSIS
27.10.3 GEOGRAPHIC PRESENCE
27.10.4 PRODUCT PORTFOLIO
27.10.5 RECENT DEVELOPMENTS
27.11 GLENMARK PHARMACEUTICALS U.S. INC.
27.11.1 COMPANY OVERVIEW
27.11.2 REVENUE ANALYSIS
27.11.3 GEOGRAPHIC PRESENCE
27.11.4 PRODUCT PORTFOLIO
27.11.5 RECENT DEVELOPMENTS
27.12 TAJ PHARMA GROUP
27.12.1 COMPANY OVERVIEW
27.12.2 REVENUE ANALYSIS
27.12.3 GEOGRAPHIC PRESENCE
27.12.4 PRODUCT PORTFOLIO
27.12.5 RECENT DEVELOPMENTS
27.13 ALKEM
27.13.1 COMPANY OVERVIEW
27.13.2 REVENUE ANALYSIS
27.13.3 GEOGRAPHIC PRESENCE
27.13.4 PRODUCT PORTFOLIO
27.13.5 RECENT DEVELOPMENTS
27.14 CONCORD BIOTECH
27.14.1 COMPANY OVERVIEW
27.14.2 REVENUE ANALYSIS
27.14.3 GEOGRAPHIC PRESENCE
27.14.4 PRODUCT PORTFOLIO
27.14.5 RECENT DEVELOPMENTS
27.15 PANACEA BIOTEC
27.15.1 COMPANY OVERVIEW
27.15.2 REVENUE ANALYSIS
27.15.3 GEOGRAPHIC PRESENCE
27.15.4 PRODUCT PORTFOLIO
27.15.5 RECENT DEVELOPMENTS
27.16 MYLAN N.V.
27.16.1 COMPANY OVERVIEW
27.16.2 REVENUE ANALYSIS
27.16.3 GEOGRAPHIC PRESENCE
27.16.4 PRODUCT PORTFOLIO
27.16.5 RECENT DEVELOPMENTS
27.17 NIKSAN PHARMACEUTICAL
27.17.1 COMPANY OVERVIEW
27.17.2 REVENUE ANALYSIS
27.17.3 GEOGRAPHIC PRESENCE
27.17.4 PRODUCT PORTFOLIO
27.17.5 RECENT DEVELOPMENTS
27.18 WELLONA PHARMA
27.18.1 COMPANY OVERVIEW
27.18.2 REVENUE ANALYSIS
27.18.3 GEOGRAPHIC PRESENCE
27.18.4 PRODUCT PORTFOLIO
27.18.5 RECENT DEVELOPMENTS
27.19 SANOFI
27.19.1 COMPANY OVERVIEW
27.19.2 REVENUE ANALYSIS
27.19.3 GEOGRAPHIC PRESENCE
27.19.4 PRODUCT PORTFOLIO
27.19.5 RECENT DEVELOPMENTS
27.2 LUPIN PHARMACEUTICALS, INC.
27.20.1 COMPANY OVERVIEW
27.20.2 REVENUE ANALYSIS
27.20.3 GEOGRAPHIC PRESENCE
27.20.4 PRODUCT PORTFOLIO
27.20.5 RECENT DEVELOPMENTS
27.21 OPELLA HEALTHCARE INTERNATIONAL SAS
27.21.1 COMPANY OVERVIEW
27.21.2 REVENUE ANALYSIS
27.21.3 GEOGRAPHIC PRESENCE
27.21.4 PRODUCT PORTFOLIO
27.21.5 RECENT DEVELOPMENTS
27.22 HEALTHY LIFE PHARMA PRIVATE LIMITED
27.22.1 COMPANY OVERVIEW
27.22.2 REVENUE ANALYSIS
27.22.3 GEOGRAPHIC PRESENCE
27.22.4 PRODUCT PORTFOLIO
27.22.5 RECENT DEVELOPMENTS
27.23 ZYDUS PHARMACEUTICALS, INC.
27.23.1 COMPANY OVERVIEW
27.23.2 REVENUE ANALYSIS
27.23.3 GEOGRAPHIC PRESENCE
27.23.4 PRODUCT PORTFOLIO
27.23.5 RECENT DEVELOPMENTS
27.24 AMNEAL PHARMACEUTICALS LLC
27.24.1 COMPANY OVERVIEW
27.24.2 REVENUE ANALYSIS
27.24.3 GEOGRAPHIC PRESENCE
27.24.4 PRODUCT PORTFOLIO
27.24.5 RECENT DEVELOPMENTS
27.25 AADI BIOSCIENCE, INC.
27.25.1 COMPANY OVERVIEW
27.25.2 REVENUE ANALYSIS
27.25.3 GEOGRAPHIC PRESENCE
27.25.4 PRODUCT PORTFOLIO
27.25.5 RECENT DEVELOPMENTS
27.26 PRESERVATION SOLUTION INC
27.26.1 COMPANY OVERVIEW
27.26.2 REVENUE ANALYSIS
27.26.3 GEOGRAPHIC PRESENCE
27.26.4 PRODUCT PORTFOLIO
27.26.5 RECENT DEVELOPMENTS
27.27 ORGAN PRESERVATION SOLUTION
27.27.1 COMPANY OVERVIEW
27.27.2 REVENUE ANALYSIS
27.27.3 GEOGRAPHIC PRESENCE
27.27.4 PRODUCT PORTFOLIO
27.27.5 RECENT DEVELOPMENTS
27.28 ORGANOX
27.28.1 COMPANY OVERVIEW
27.28.2 REVENUE ANALYSIS
27.28.3 GEOGRAPHIC PRESENCE
27.28.4 PRODUCT PORTFOLIO
27.28.5 RECENT DEVELOPMENTS
27.29 OPSL GROUP
27.29.1 COMPANY OVERVIEW
27.29.2 REVENUE ANALYSIS
27.29.3 GEOGRAPHIC PRESENCE
27.29.4 PRODUCT PORTFOLIO
27.29.5 RECENT DEVELOPMENTS
27.3 XVIVO PERFUSION
27.30.1 COMPANY OVERVIEW
27.30.2 REVENUE ANALYSIS
27.30.3 GEOGRAPHIC PRESENCE
27.30.4 PRODUCT PORTFOLIO
27.30.5 RECENT DEVELOPMENTS
27.31 SANDOR
27.31.1 COMPANY OVERVIEW
27.31.2 REVENUE ANALYSIS
27.31.3 GEOGRAPHIC PRESENCE
27.31.4 PRODUCT PORTFOLIO
27.31.5 RECENT DEVELOPMENTS
27.32 PARAGONIX TECHNOLOGIES
27.32.1 COMPANY OVERVIEW
27.32.2 REVENUE ANALYSIS
27.32.3 GEOGRAPHIC PRESENCE
27.32.4 PRODUCT PORTFOLIO
27.32.5 RECENT DEVELOPMENTS
27.33 AOPO ORGANIZATION
27.33.1 COMPANY OVERVIEW
27.33.2 REVENUE ANALYSIS
27.33.3 GEOGRAPHIC PRESENCE
27.33.4 PRODUCT PORTFOLIO
27.33.5 RECENT DEVELOPMENTS
27.34 CARDIOLINK GROUP
27.34.1 COMPANY OVERVIEW
27.34.2 REVENUE ANALYSIS
27.34.3 GEOGRAPHIC PRESENCE
27.34.4 PRODUCT PORTFOLIO
27.34.5 RECENT DEVELOPMENTS
27.35 ESSENTIAL PHARMACEUTICALS LLC
27.35.1 COMPANY OVERVIEW
27.35.2 REVENUE ANALYSIS
27.35.3 GEOGRAPHIC PRESENCE
27.35.4 PRODUCT PORTFOLIO
27.35.5 RECENT DEVELOPMENTS
27.36 TRANSMEDICS
27.36.1 COMPANY OVERVIEW
27.36.2 REVENUE ANALYSIS
27.36.3 GEOGRAPHIC PRESENCE
27.36.4 PRODUCT PORTFOLIO
27.36.5 RECENT DEVELOPMENTS
27.37 SALF SPA
27.37.1 COMPANY OVERVIEW
27.37.2 REVENUE ANALYSIS
27.37.3 GEOGRAPHIC PRESENCE
27.37.4 PRODUCT PORTFOLIO
27.37.5 RECENT DEVELOPMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
28 RELATED REPORTS
29 CONCLUSION
30 QUESTIONNAIRE
31 ABOUT DATA BRIDGE MARKET RESEARCH
研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。













