Global Low Grade Glioma Market
حجم السوق بالمليار دولار أمريكي
CAGR :
% 
 USD
1.11 Billion
USD
1.55 Billion
2024
2032
USD
1.11 Billion
USD
1.55 Billion
2024
2032
| 2025 –2032 | |
| USD 1.11 Billion | |
| USD 1.55 Billion | |
|
|
|
|
Global Low-Grade Glioma Market Segmentation, By Type (Low-grade I and Low-grade II), Drug Class (Trametinib, Dabrafenib, Ivosidenib and Mirdametinib), Route of Administration (Topical and Oral), Distribution Channel (Online Pharmacies, Hospital Pharmacies and Retail Pharmacies) – Industry Trends and Forecast to 2032
Low-Grade Glioma Market Analysis
The low-grade glioma market has experienced significant growth, driven by advancements in diagnostics and treatments. With a growing emphasis on early detection and personalized therapies, there is increasing demand for effective treatment options, including surgery, radiation therapy, and chemotherapy. Innovations in targeted therapies and immunotherapies have further fueled market expansion, offering more precise treatment modalities with fewer side effects compared to traditional methods.
The market is also influenced by the rising prevalence of low-grade gliomas and growing awareness among healthcare providers and patients about available treatment options. Increased research and development investments, along with collaborations between pharmaceutical companies, biotech firms, and academic institutions, have accelerated the introduction of novel therapies to the market. Furthermore, clinical trials exploring new drug candidates and treatment combinations are expected to further shape market dynamics.
The ongoing development of better diagnostic tools, such as advanced imaging techniques, is improving the accuracy of low-grade glioma diagnoses, facilitating earlier intervention and better patient outcomes. However, challenges such as high treatment costs, the complexity of treatment regimens, and limited availability of specialized healthcare infrastructure in certain regions may hinder market growth to some extent. Despite these challenges, the overall outlook for the low-grade glioma market remains positive, with continued advancements expected to improve patient care and treatment efficacy.
Low-Grade Glioma Market Size
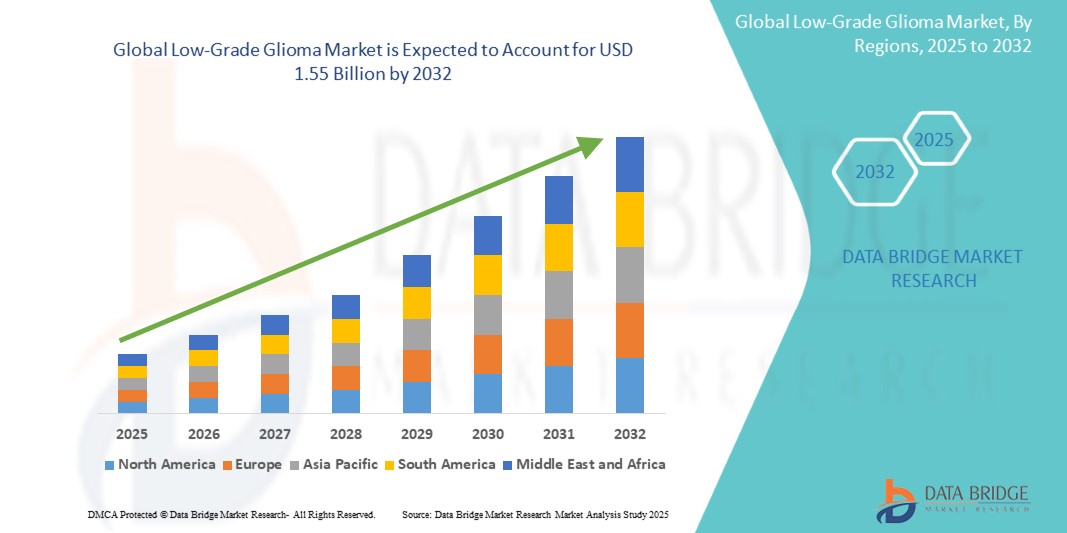
The global Low-Grade Glioma market size was valued at USD 1.11 billion in 2024 and is projected to reach USD 1.55 billion by 2032, with a CAGR of 4.28% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Low-Grade Glioma Market Trends
“Increasing Adoption of Targeted Therapies and Immunotherapies”
One major trend in the low-grade glioma market is the increasing adoption of targeted therapies and immunotherapies. These treatments are gaining traction as they offer more precise and effective options compared to traditional therapies such as chemotherapy and radiation. Targeted therapies work by focusing on specific genetic mutations or molecular targets present in glioma cells, minimizing damage to surrounding healthy tissue. Immunotherapies, such as checkpoint inhibitors and vaccine-based treatments, aim to enhance the body's immune system to recognize and destroy cancer cells.
The growing understanding of the genetic and molecular underpinnings of low-grade gliomas has accelerated the development of these advanced therapies, offering the potential for improved patient outcomes and reduced side effects. Ongoing clinical trials and research into these innovative treatments are expected to further shape the market, making this trend a key driver in the future of low-grade glioma care and treatment strategies.
Report Scope and Low-Grade Glioma Market Segmentation
|
Attributes |
Low-Grade Glioma Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America |
|
Key Market Players |
AstraZeneca (UK), AbbVie Inc. (U.S.), Amgen Inc. (U.S.), Bristol-Myers Squibb Company (U.S.), DAIICHI SANKYO COMPANY, LIMITED (Japan), Day One Biopharmaceuticals, Inc. (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), Genentech, Inc. (U.S.), Helsinn Healthcare SA (Switzerland), Lilly (U.S.), LES LABORATOIRES SERVIER (France), Merck & Co., Inc. (U.S.), Merck KGaA (Germany), Novo Nordisk A/S (Denmark), Novartis AG (Switzerland), Pfizer Inc. (U.S.), Rigel Pharmaceuticals, Inc. (U.S.), SpringWorks Therapeutics, Inc. (U.S.), Sun Pharmaceutical Industries Ltd. (India) and Teva Pharmaceutical Industries Ltd. (Israel) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Low-Grade Glioma Market Definition
Low-grade glioma refers to a type of brain tumor that originates in the glial cells, which support and protect nerve cells in the brain. These tumors are typically classified as grade 1 or grade 2 based on their relatively slow growth and lower malignancy compared to higher-grade gliomas. Low-grade gliomas are more common in younger patients and may occur in various parts of the brain. While they are less aggressive than high-grade gliomas, they can still cause significant neurological symptoms and require treatment such as surgery, radiation, and sometimes chemotherapy. Because of their slow growth, low-grade gliomas can be challenging to detect early but may evolve into more aggressive forms over time.
Low-Grade Glioma Market Dynamics
Drivers
- Advancements in Targeted Therapies and Immunotherapies
The increasing development and adoption of targeted therapies and immunotherapies are significant drivers of the low-grade glioma market. Targeted therapies focus on specific genetic mutations or proteins involved in tumor growth, offering more precise treatments with fewer side effects compared to traditional methods such as chemotherapy and radiation. Immunotherapies, such as checkpoint inhibitors, work by boosting the body's immune system to better target cancer cells. A prime instance is the development of drugs such as mirdametinib, an investigational MEK inhibitor, which is being evaluated for low-grade gliomas in clinical trials. These advanced treatments hold the potential to improve patient outcomes significantly by offering more personalized and effective options. As these therapies become more widely available, they are expected to revolutionize the treatment landscape for low-grade gliomas, driving market growth by improving survival rates and reducing treatment-related side effects. The growing interest and investment in these therapies are expected to increase market size as demand for targeted treatments rises, contributing to the overall shift toward more specialized and effective treatment regimens.
- Increase in Early Diagnosis and Improved Imaging Technologies
The rise in early diagnosis due to advances in imaging technologies is another critical factor driving the low-grade glioma market. More accurate diagnostic tools, such as advanced MRI scans, positron emission tomography (PET), and functional imaging techniques, enable earlier detection of gliomas, which can lead to more timely interventions. The ability to detect low-grade gliomas at earlier stages allows for better treatment outcomes, reducing tumor progression and enhancing the quality of life for patients. For instance, sophisticated imaging techniques help in differentiating between tumor types, facilitating precise treatment planning. Moreover, early diagnosis can help identify patients who are most likely to benefit from emerging therapies, such as targeted treatments or immunotherapy. As diagnostic tools improve, the market for low-grade glioma treatments expands, with earlier intervention leading to increased demand for therapies, particularly those aimed at long-term disease management and monitoring. This contributes to market growth by promoting a shift toward proactive care strategies.
Opportunities
- Growth in Pediatric and Adolescent Treatment Options
One significant opportunity in the low-grade glioma market lies in expanding treatment options for pediatric and adolescent patients. Low-grade gliomas are one of the most common brain tumors in children and young adults, and there is a notable gap in personalized treatment options for this age group. In response, pharmaceutical companies are increasingly focusing on developing therapies specifically tailored to younger patients, who often require different dosing regimens and experience distinct side effects compared to adults. For instance, SpringWorks Therapeutics initiated a Phase 1/2 trial in 2021 to assess mirdametinib, an investigational MEK inhibitor, in children and young adults with low-grade glioma. This focus on pediatric and adolescent care offers a significant market opportunity, as the demand for age-appropriate therapies grows. As these specialized treatments gain approval, they will likely increase market penetration and attract new investments in the low-grade glioma treatment landscape.
- Expansion of Clinical Trials for New Treatment Modalities
The growing number of clinical trials evaluating innovative therapies for low-grade gliomas represents a key opportunity in the market. With increasing research into new treatment modalities such as gene therapies, oncolytic viruses, and combination therapies, clinical trials are driving the development of more effective and personalized treatment strategies. For instance, Mustang Bio's Orphan Drug Designation for MB-108, an HSV-1 oncolytic virus for malignant glioma, reflects the increasing interest in alternative therapies for gliomas. The expansion of clinical trials will likely lead to the introduction of breakthrough treatments, broadening the range of available therapies for low-grade glioma patients. This growth in treatment options can attract both investment and demand, fueling the overall expansion of the market as new therapies are approved and integrated into standard care practices.
Restraints/Challenges
- High Treatment Costs
One of the primary restraints in the low-grade glioma market is the high cost of treatment, particularly for advanced therapies such as targeted treatments and immunotherapies. These therapies, although more effective, can be significantly more expensive compared to traditional methods such as surgery and chemotherapy. For instance, the use of mirdametinib, a novel MEK inhibitor, may come with a higher price tag due to its investigational status and specialized nature. The high cost of treatment can limit access for patients, particularly in regions with less healthcare infrastructure or for those without sufficient insurance coverage. The financial burden associated with these treatments could hinder market expansion, as not all patients will be able to afford cutting-edge therapies. Pharmaceutical companies and healthcare systems may face pressure to find ways to reduce costs or offer financial assistance programs to ensure wider accessibility, thereby influencing market growth potential.
- Tumor Resistance and Disease Progression
A significant challenge in treating low-grade gliomas is the potential for tumor resistance and progression over time. While low-grade gliomas are initially slow-growing, they can evolve into more aggressive, high-grade gliomas, which are more difficult to treat. This progression can limit the long-term effectiveness of treatments and make it difficult to manage the disease in the long run. For instance, despite the promising results of targeted therapies such as mirdametinib, resistance to such treatments may develop, rendering them less effective. The challenge of tumor resistance and progression can limit the sustained effectiveness of available treatments, potentially slowing down the adoption of new therapies.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Low-Grade Glioma Market Scope
The market is segmented on the basis of type, drug class, route of administration and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Low-grade I
- Low-grade II
Drug Class
- Trametinib
- Dabrafenib
- Ivosidenib
- Mirdametinib
Route of Administration
- Topical
- Oral
Distribution Channel
- Online Pharmacies
- Hospital Pharmacies
- Retail Pharmacies
Low-Grade Glioma Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, type, drug class, route of administration and distribution channel as referenced above.
The countries covered in the market report are U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America.
North America is expected to dominate the low-grade glioma market due to its advanced healthcare infrastructure, high prevalence of brain cancer, and strong research and development investments. The region benefits from cutting-edge diagnostic technologies and novel treatment options, including targeted therapies and immunotherapies. In addition, a high level of awareness, early diagnosis, and the presence of major pharmaceutical and biotech companies conducting clinical trials further contribute to North America's leadership in the market.
Asia Pacific is expected to exhibit the highest growth rate in the low-grade glioma market. This growth is driven by improving healthcare infrastructure, increasing cancer awareness, and expanding access to advanced treatments in countries such as China and India. In addition, rising research and development investments, along with growing healthcare expenditures, are contributing to market expansion. As early diagnosis and treatment options become more accessible, the demand for low-grade glioma therapies is expected to surge in this region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Low-Grade Glioma Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Low-Grade Glioma Market Leaders Operating in the Market Are:
- AstraZeneca (UK)
- AbbVie Inc. (U.S.)
- Amgen Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- DAIICHI SANKYO COMPANY, LIMITED (Japan)
- Day One Biopharmaceuticals, Inc. (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Genentech, Inc. (U.S.)
- Helsinn Healthcare SA (Switzerland)
- Lilly (U.S.)
- LES LABORATOIRES SERVIER (France)
- Merck & Co., Inc. (U.S.)
- Merck KGaA (Germany)
- Novo Nordisk A/S (Denmark)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- Rigel Pharmaceuticals, Inc. (U.S.)
- SpringWorks Therapeutics, Inc. (U.S.)
- Sun Pharmaceutical Industries Ltd. (India)
- Teva Pharmaceutical Industries Ltd. (Israel)
Latest Developments in Low-Grade Glioma Market
- In November 2024, Mustang Bio, Inc. announced that the U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation to MB-108, a herpes simplex virus type 1 (HSV-1) oncolytic virus, for the treatment of malignant glioma
- In September 2024, Adaptin Bio announced that the FDA had cleared an Investigational New Drug (IND) application for its APTN-101 program in glioblastoma (GBM), the most prevalent and aggressive primary brain tumor. This clearance allows the company to begin a first-in-human Phase 1 clinical trial to assess the investigational candidate's potential in treating GBM
- In August 2024, Servier announced that the U.S. Food and Drug Administration (FDA) has approved VORANIGO, an inhibitor targeting isocitrate dehydrogenase-1 (IDH1) and isocitrate dehydrogenase-2 (IDH2). This treatment is now approved for adult and pediatric patients aged 12 and older with Grade 2 astrocytoma or oligodendroglioma that carry a susceptible IDH1 or IDH2 mutation, following surgery such as biopsy, subtotal resection, or gross total resection. VORANIGO is now available, providing glioma patients with a convenient once-daily pill to actively manage their disease
- In September 2023, AnHeart Therapeutics announced that the first patient had been dosed in the Phase 2 clinical trial, G203, which is assessing the effectiveness of safusidenib in patients with Grades 2 or 3 recurrent or progressive mutant isocitrate dehydrogenase 1 (mIDH1) glioma, a prevalent form of adult primary brain cancer
- In June 2021, SpringWorks Therapeutics, Inc. announced the launch of a Phase 1/2 clinical trial to assess the efficacy of mirdametinib, an experimental MEK inhibitor, in treating children, adolescents, and young adults with low-grade glioma (LGG)
SKU-
احصل على إمكانية الوصول عبر الإنترنت إلى التقرير الخاص بأول سحابة استخبارات سوقية في العالم
- لوحة معلومات تحليل البيانات التفاعلية
- لوحة معلومات تحليل الشركة للفرص ذات إمكانات النمو العالية
- إمكانية وصول محلل الأبحاث للتخصيص والاستعلامات
- تحليل المنافسين باستخدام لوحة معلومات تفاعلية
- آخر الأخبار والتحديثات وتحليل الاتجاهات
- استغل قوة تحليل المعايير لتتبع المنافسين بشكل شامل
منهجية البحث
يتم جمع البيانات وتحليل سنة الأساس باستخدام وحدات جمع البيانات ذات أحجام العينات الكبيرة. تتضمن المرحلة الحصول على معلومات السوق أو البيانات ذات الصلة من خلال مصادر واستراتيجيات مختلفة. تتضمن فحص وتخطيط جميع البيانات المكتسبة من الماضي مسبقًا. كما تتضمن فحص التناقضات في المعلومات التي شوهدت عبر مصادر المعلومات المختلفة. يتم تحليل بيانات السوق وتقديرها باستخدام نماذج إحصائية ومتماسكة للسوق. كما أن تحليل حصة السوق وتحليل الاتجاهات الرئيسية هي عوامل النجاح الرئيسية في تقرير السوق. لمعرفة المزيد، يرجى طلب مكالمة محلل أو إرسال استفسارك.
منهجية البحث الرئيسية التي يستخدمها فريق بحث DBMR هي التثليث البيانات والتي تتضمن استخراج البيانات وتحليل تأثير متغيرات البيانات على السوق والتحقق الأولي (من قبل خبراء الصناعة). تتضمن نماذج البيانات شبكة تحديد موقف البائعين، وتحليل خط زمني للسوق، ونظرة عامة على السوق ودليل، وشبكة تحديد موقف الشركة، وتحليل براءات الاختراع، وتحليل التسعير، وتحليل حصة الشركة في السوق، ومعايير القياس، وتحليل حصة البائعين على المستوى العالمي مقابل الإقليمي. لمعرفة المزيد عن منهجية البحث، أرسل استفسارًا للتحدث إلى خبراء الصناعة لدينا.
التخصيص متاح
تعد Data Bridge Market Research رائدة في مجال البحوث التكوينية المتقدمة. ونحن نفخر بخدمة عملائنا الحاليين والجدد بالبيانات والتحليلات التي تتطابق مع هدفهم. ويمكن تخصيص التقرير ليشمل تحليل اتجاه الأسعار للعلامات التجارية المستهدفة وفهم السوق في بلدان إضافية (اطلب قائمة البلدان)، وبيانات نتائج التجارب السريرية، ومراجعة الأدبيات، وتحليل السوق المجدد وقاعدة المنتج. ويمكن تحليل تحليل السوق للمنافسين المستهدفين من التحليل القائم على التكنولوجيا إلى استراتيجيات محفظة السوق. ويمكننا إضافة عدد كبير من المنافسين الذين تحتاج إلى بيانات عنهم بالتنسيق وأسلوب البيانات الذي تبحث عنه. ويمكن لفريق المحللين لدينا أيضًا تزويدك بالبيانات في ملفات Excel الخام أو جداول البيانات المحورية (كتاب الحقائق) أو مساعدتك في إنشاء عروض تقديمية من مجموعات البيانات المتوفرة في التقرير.