A transmembrane glycoprotein called PSMA, found on the cell membrane, is specifically and prominently expressed in prostate cancer (PCa). Additionally, tumor invasiveness is correlated with its expression level. Over the past 20 years, much research has been done on PSMA as a molecular target of PCa. There is currently a lot of evidence points to major advancements in PCa PSMA-targeted therapy. The discovery of new PSMA-targeting drugs for diagnostic and therapeutic purposes has exploded over the past five years. PSMA-targeting imaging agents have been created for SPECT and PET platforms. In cases of advanced disease, biochemical recurrence following treatment, and high-risk localized disease, PSMA PET imaging seems to perform better than conventional imaging.
Prostate Cancer: The Wrath in the World
In the world, prostate cancer is a severe issue for public health. In 2020, it was the second most prevalent cancer and the fifth most common cause of cancer mortality in men. This cancer develops in prostatic tissues (a gland in the male reproductive system found below the bladder and in front of the rectum). The urethra, the channel through which pee travels, is encircled by the prostate. The size of a healthy prostate is comparable to a walnut. The urethra becomes compressed if the prostate enlarges too much. The usual flow of urine may be slowed or stopped as a result. In older men, prostate cancer most frequently arises.
The Prevalence of Prostate Cancer
Prostate cancer is the most prevalent cancer in American men, except for skin cancer. According to projections from the American Cancer Society, there will be an estimated:
-
268,490 new cases of prostate cancer are estimated in 2023
-
Prostate cancer causes about 34,500 deaths annually
Prostate Cancer Risk
Prostate cancer will be detected in about 1 in every 8 men at some point in their lifetime.
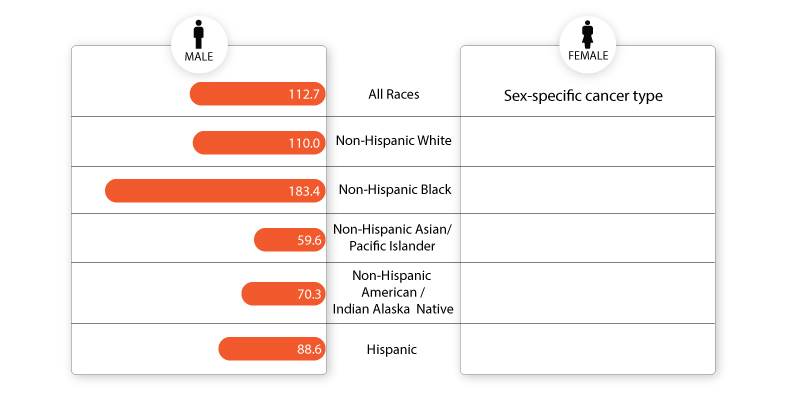
Older males and non-Hispanic Black men have a higher risk of developing prostate cancer.
It is uncommon in men under 40 and is diagnosed in about 6 out of 10 men who are 65 or older.
The typical diagnostic age for men is 66.
Fig.1: Rate of new cases per 100,000 persons by race/ethnicity: prostate cancer
Source: SEER 22 2015–2019
Deaths from Prostate Cancer
Behind lung cancer, prostate cancer is the second most common cancer among American males. Prostate cancer claims the lives of about 1 in 41 men.
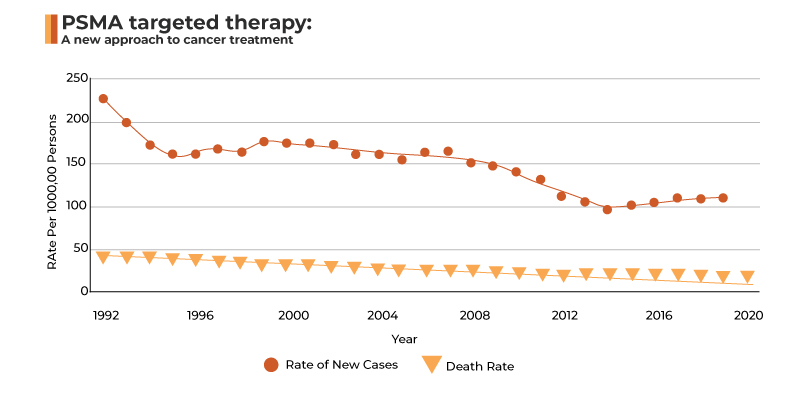
Fig.1: Prostate cancer at glance
Source: National Cancer Institute
How do Targeted Therapies Work?
Before understanding the mechanism of PSMA-targeted therapy, it is essential to comprehend the concept of targeted therapy in general.
Targeted therapy drugs for prostate cancer precisely attack cancer cells and stop them from growing and spreading. They give you a personalized type of treatment based on your particular cancer’s genetic makeup.
Targeted therapy medicines zero in on molecules that control some of the functions of cancer cells. Different targeted drugs work in different ways. They may affect how cancer cells divide, grow, interact with other cells, or repair themselves. Some harness the power of your immune system to fight cancer.
This kind of treatment is different from chemotherapy because it mostly spares healthy cells, which are often damaged along with cancer cells by chemotherapy. Targeted therapies are designed to stop only the growth of cells with a certain mutation.
PSMA-Directed Therapies and their Scope in Prostate Cancer
The ability to detect and treat prostate cancer has been transformed by developing therapies targeting PSMA, a cell surface transmembrane protein. This cell surface transmembrane protein is overexpressed in most prostate cancer cells, particularly in castration-resistant diseases. Gallium 68 PSMA-11 (Ga 68 PSMA-11), the first agent approved for positron emission tomography (PET) imaging of prostate-specific membrane antigen (PSMA)-positive lesions in patients with prostate cancer, was approved in December 2020, ushering in an exciting new chapter in the diagnosis and treatment of metastatic castration-resistant prostate cancer (mCRPC).
The ability to detect and treat prostate cancer has been transformed by developing therapies that target PSMA, a cell surface transmembrane protein. This cell surface transmembrane protein is overexpressed in most prostate cancer cells, particularly in castration-resistant diseases. PSMA-directed therapeutics are a growing field. Many of these therapeutics are linked to a PSMA scan in order to identify patients who are most likely to respond to those therapeutics because the presence of the molecule that the therapeutic will be targeting can be established. The majority of PSMA-directed therapeutic trials use a PSMA scan prior to treatment to try to select patients who are likely to respond.
Additionally, PSMA seems to have a significant part in theranostics. Although there are more and more drugs and treatments for metastatic castration-resistant prostate cancer (mCRPC), the median survival time for patients who have never received chemotherapy is only about 31 to 35 months. Lu-PSMA, a small-molecule PSMA inhibitor, and other PSMA radioligands have recently shown promise in meeting this demand. Despite the availability of numerous radioligands, Lu-PSMA has the favored pharmacokinetic profile because of its minimal toxicity and decreased kidney absorption.
PSMA-Cost Prohibitive or Cost-Effective?
Although the diagnostic and therapeutic potential of PSMA is encouraging, particularly given the expanding number of clinical applications, treatment expense must also be considered when determining applicability. Using data from the proPSMA study and prospectively established key inputs, Cardet et al. conducted a cost-effectiveness analysis for PSMA as a diagnostic tool to evaluate the costs of patients with high-risk prostate cancer who underwent conventional imaging against Ga-PSMA PET/CT. According to the data, the upfront cost of PSMA PET/CT is roughly equivalent to traditional imaging for the identification of metastatic disease ($1,140 versus $1,181 AUD). The best cost-effective method for detecting PSMA remained PSMA PET/CT because it was less expensive and more accurate when the cost per scan and total accuracy of detection were combined.
Gordon et al. used a decision-analytic model using Markov modeling chains to conduct another cost-effectiveness analysis of Ga-PSMA PET/CT as a modality to detect prostate cancer recurrence. Costs to the health system and years of survival over 10 years were the main outcomes that were measured. The cost model was sensitive to the proportion of patients with correctly discovered Ga-PSMA prostate cancer lesions, the price of standard medical care, and Ga-PSMA follow-up studies. Ga-PSMA maintained its superiority in terms of cost savings and longer life years across all values assessed.
Adopting 68Ga-PSMA as the standard of treatment for the identification of recurrent prostate cancer merits consideration given the research mentioned above' findings that it is cost-effective, or at the very least, cost-neutral. Cost analysis studies still need to be carried out in relation to the first identification of prostate cancer. However, considering its diagnostic efficacy, it will probably also be reasonably priced.
The Future
An interesting and developing field of study is the diagnostic and therapeutic role of PSMA. For individuals with biochemical prostate cancer recurrence, this new cutting-edge molecular imaging technique seems more effective and could be less expensive than the current standard of care. High-quality data and a well-done cost study have enhanced the enthusiasm for this new medical technology. Although there is no universal agreement on its use as an initial diagnostic tool or ligand therapy, the evidence at hand suggests that it will likely meet these unmet needs in the management of prostate cancer, and the NCCN has already included it in its prostate cancer guidelines.