Europe Postpartum Hemorrhage Treatment Devices Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
274.95 Million
USD
397.15 Million
2024
2032
USD
274.95 Million
USD
397.15 Million
2024
2032
| 2025 –2032 | |
| USD 274.95 Million | |
| USD 397.15 Million | |
|
|
|
|
Europäischer Markt für Geräte zur Behandlung postpartaler Blutungen, Typ (Uterusballontamponade, vorgefülltes Uniject-Injektionssystem, nicht-pneumatisches Anti-Schock-Kleidungsstück, Geräte zur vakuuminduzierten Blutungskontrolle und andere), Zustand (starke postpartale Blutung (mehr als 1000 ml), leichte postpartale Blutung (500-1000 ml), massive postpartale Blutung (2000 ml oder mehr) und sekundäre postpartale Blutung), Patiententyp (primäre PPH und sekundäre PPH), Endbenutzer (Krankenhäuser, Entbindungszentren, Fachkliniken, Einrichtungen der häuslichen Pflege und andere), Vertriebskanal (Direktausschreibung, Einzelhandel und andere) – Branchentrends und Prognose bis 2032
Geräte zur Behandlung postpartaler Blutungen Marktgröße
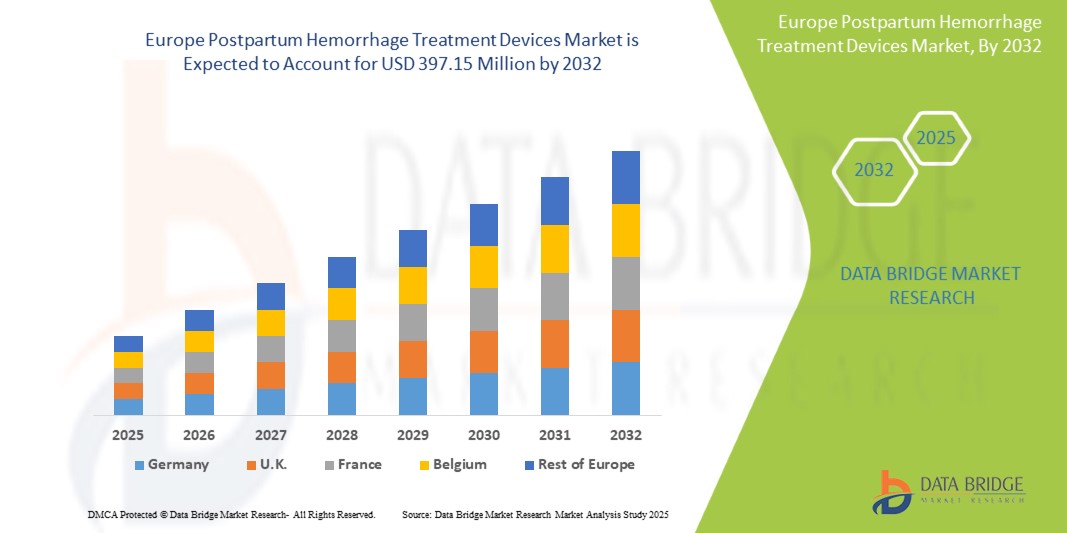
- Der europäische Markt für Geräte zur Behandlung postpartaler Blutungen wurde im Jahr 2024 auf 274,95 Millionen US-Dollar geschätzt und soll bis 2032 397,15 Millionen US-Dollar erreichen.
- Im Prognosezeitraum von 2025 bis 2032 dürfte der Markt mit einer jährlichen Wachstumsrate von 4,7 % wachsen, vor allem aufgrund der steigenden Zahl postpartaler Blutungen.
- Zu den wichtigsten Treibern des Marktes für Geräte zur Behandlung postpartaler Blutungen zählen die zunehmende Häufigkeit postpartaler Blutungen, das zunehmende Bewusstsein für wirksame Behandlungsmöglichkeiten und technologische Fortschritte.
Analyse von Geräten zur Behandlung postpartaler Blutungen
- Die zunehmende Zahl postpartaler Blutungen, die auf Faktoren wie die steigende Zahl von Kaiserschnitten und Geburtskomplikationen zurückzuführen ist, hat zu einer steigenden Nachfrage nach wirksamen Behandlungsgeräten geführt.
- Innovationen in der Medizintechnik, darunter neuere Uterustamponaden, blutstillende Mittel und minimalinvasive chirurgische Optionen, tragen zu einer Verbesserung der Behandlungsergebnisse bei und treiben das Marktwachstum voran.
- Beispielsweise variiert der Markt für PPH-Behandlungsgeräte geografisch, wobei in Entwicklungsregionen aufgrund steigender Investitionen im Gesundheitswesen und der Konzentration auf die Gesundheit von Müttern ein signifikantes Wachstum zu beobachten ist.
- Daher weist der Markt für PPH-Behandlungsgeräte eine starke geografische Ungleichheit auf, wobei Entwicklungsregionen aufgrund steigender Investitionen im Gesundheitswesen und eines gezielten Ansatzes zur Verbesserung der Gesundheit von Müttern ein erhebliches Wachstum verzeichnen.
Berichtsumfang und Segmentierung von Geräten zur Behandlung postpartaler Blutungen
|
Eigenschaften |
Wichtige Markteinblicke zu Geräten zur Behandlung postpartaler Blutungen |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
Europa
|
|
Wichtige Marktteilnehmer |
|
|
Marktchancen |
|
|
Wertschöpfungsdaten-Infosets |
Zusätzlich zu den Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und wichtige Akteure enthalten die von Data Bridge Market Research kuratierten Marktberichte auch Import-Export-Analysen, eine Übersicht über die Produktionskapazität, eine Analyse des Produktionsverbrauchs, eine Preistrendanalyse, ein Szenario des Klimawandels, eine Lieferkettenanalyse, eine Wertschöpfungskettenanalyse, eine Übersicht über Rohstoffe/Verbrauchsmaterialien, Kriterien für die Lieferantenauswahl, eine PESTLE-Analyse, eine Porter-Analyse und regulatorische Rahmenbedingungen. |
Markttrends für Geräte zur Behandlung postpartaler Blutungen
„Zunehmende Nutzung von Uterusballontamponaden“
- Uterusballontamponaden dienen der Kontrolle der PPH, indem sie direkten Druck auf die Gebärmutterwand ausüben und so die Hämostase fördern. Ihre Wirksamkeit im klinischen Umfeld ist umfassend dokumentiert, was zu einem gestiegenen Vertrauen der Gesundheitsdienstleister in den Einsatz dieser Geräte als Erstlinienintervention zur Behandlung der PPH und damit zu ihrer zunehmenden Akzeptanz geführt hat.
- So gab es beispielsweise im Gesundheitswesen konzertierte Anstrengungen, die Ausbildung und Aufklärung im Umgang mit PPH zu verbessern. Dazu gehört auch eine spezielle Schulung zur Anwendung von Uterusballontamponaden. Das gestiegene Bewusstsein des medizinischen Fachpersonals für die Bedeutung eines schnellen Eingreifens hat zu einer breiteren Akzeptanz und Nutzung dieser Geräte sowohl in Industrie- als auch in Entwicklungsländern geführt.
- Uterusballontamponaden werden zunehmend in umfassende Protokolle zur Mütterbetreuung integriert, insbesondere in der Notfallgeburtshilfe.
- Diese Integration wird durch Richtlinien von Organisationen wie der WHO und verschiedenen Initiativen für Müttergesundheit unterstützt, die evidenzbasierte Praktiken zur effektiven Prävention und Behandlung von PPH fördern. Die Ausrichtung dieser Geräte an klinischen Richtlinien erleichtert ihre breite Einführung in Krankenhäusern und Geburtszentren.
Treiber
„ Steigende Häufigkeit postpartaler Blutungen “
- Die zunehmende Zahl postpartaler Blutungen (PPH) hat einen dringenden Bedarf an wirksamen Behandlungsgeräten auf dem Weltmarkt geschaffen. Ein gesteigertes Bewusstsein für mütterliche Gesundheitsprobleme und eine bessere Identifizierung gefährdeter Bevölkerungsgruppen haben zu einem Anstieg der Nachfrage nach innovativen Lösungen geführt.
- Da die Sicherheit der Mutter im Gesundheitswesen oberste Priorität hat, werden zunehmend fortschrittliche medizinische Geräte eingesetzt, die der Vorbeugung oder Behandlung von PPH dienen, darunter Geräte zur Gebärmutterkompression und blutstillende Mittel.
Zum Beispiel,
- Laut NCBI war die postpartale Hämorrhagie (PPH) im Mai 2020 weltweit die häufigste Ursache für Müttersterblichkeit. In den USA stieg die PPH um 26 %. Dieser alarmierende Anstieg der Fälle unterstreicht die entscheidende Bedeutung der Bekämpfung der PPH und dient als Katalysator für verstärkte Investitionen in innovative Behandlungslösungen und Technologien zur Prävention und Behandlung dieser potenziell lebensbedrohlichen Erkrankung.
- Im August 2024 erklärte die Weltgesundheitsorganisation, dass die postpartale Hämorrhagie (PPH), allgemein definiert als Blutverlust von 500 ml oder mehr innerhalb von 24 Stunden nach der Geburt, weltweit die häufigste Ursache für Müttersterblichkeit ist. Sie betrifft jedes Jahr Millionen von Frauen und ist für über 20 % aller weltweit gemeldeten Müttersterbefälle verantwortlich.
Gelegenheiten
„ Schulungs- und Ausbildungsprogramme für die ordnungsgemäße Verwendung von PPH-Behandlungsgeräten “
- Die Entwicklung umfassender Schulungs- und Ausbildungsprogramme für den richtigen Einsatz von Geräten zur Behandlung von PPH (postpartale Hämorrhagien) bietet eine wertvolle Gelegenheit, die Gesundheitsversorgung von Müttern zu stärken.
- Diese Programme können auf Mitarbeiter im Gesundheitswesen auf verschiedenen Ebenen zugeschnitten werden, von Gemeindegesundheitsarbeitern bis hin zu qualifizierten Geburtshelfern und Krankenhauspersonal. So wird sichergestellt, dass alle an der Mutterschaftsfürsorge beteiligten Mitarbeiter über das notwendige Wissen und die praktische Erfahrung verfügen.
Einschränkungen/Herausforderungen
„ Umweltauswirkungen und Entsorgungsprobleme von PPH-Geräten zum Einmalgebrauch “
- Einwegprodukte zur Behandlung postpartaler Blutungen (PPH), wie Uterusballontamponaden und Absaugsysteme, erzeugen erhebliche Mengen medizinischen Abfalls und stellen insbesondere in ressourcenarmen Regionen eine Herausforderung für Umwelt und Entsorgung dar. Ohne eine entsprechende Abfallwirtschaftsinfrastruktur können sich entsorgte Produkte auf Mülldeponien oder offenen Müllhalden ansammeln und schädliche Materialien wie Kunststoffe und biologisch gefährliche Rückstände in die Umwelt freisetzen.
- Unsachgemäße Entsorgung erhöht zudem das Risiko, kontaminierte Geräte in Notsituationen zu entsorgen oder wiederzuverwenden. Dies erhöht das Infektionsrisiko für Patienten und medizinisches Personal, beispielsweise durch Hepatitis oder Sepsis. Diese doppelte Bedrohung – Umweltschäden und Gesundheitsrisiken – unterstreicht die Notwendigkeit nachhaltiger Design- und Entsorgungslösungen bei der Entwicklung von PPH-Geräten.
Zum Beispiel,
- Im März 2022 wies MDPI darauf hin, dass die zunehmende Verwendung von Einweg-Geräten zur postpartalen Blutungsbehandlung das steigende Volumen an Krankenhausabfällen erhöht, von denen viele gefährlich sind und zu Infektionsrisiken und Umweltverschmutzung beitragen. Unsachgemäße Entsorgung und die zunehmende Verwendung von Einwegartikeln wie Spritzen und Kathetern erfordern dringende Aufmerksamkeit für ein nachhaltiges Abfallmanagement in der Müttergesundheitsversorgung.
- Laut Centurial vom Dezember 2024 erhöhen Einweg-PPH-Behandlungsgeräte zwar die Sicherheit und verringern das Infektionsrisiko, ihre weit verbreitete Verwendung wirft jedoch Umweltbedenken auf. Unsachgemäße Entsorgung trägt zur Ansammlung medizinischer Abfälle und zur Umweltverschmutzung bei. Die Abwägung des Bedarfs an sterilen Einweginstrumenten mit einem nachhaltigen Abfallmanagement ist unerlässlich, um Umweltschäden zu minimieren und gleichzeitig eine effektive Mutterschaftsfürsorge zu gewährleisten.
Europa Marktumfang für Geräte zur Behandlung postpartaler Blutungen
Der europäische Markt für Geräte zur Behandlung postpartaler Blutungen ist in fünf wichtige Segmente unterteilt, die auf Typ, Zustand, Patiententyp, Endbenutzer und Vertriebskanal basieren.
|
Segmentierung |
Untersegmentierung |
|
Nach Typ |
|
|
Nach Bedingung |
|
|
Nach Patiententyp |
|
|
Nach Endbenutzer
|
|
|
Nach Vertriebskanal |
|
Europa Markt für Geräte zur Behandlung postpartaler Blutungen – Regionale Analyse
„Deutschland ist das dominierende Land auf dem Markt für Geräte zur Behandlung postpartaler Blutungen “
- Deutschland verfügt über ein gut etabliertes Gesundheitssystem mit hohen Standards in der Mütterversorgung. Die proaktive Politik der Regierung und die erheblichen Investitionen in die Müttergesundheit tragen dazu bei, dass fortschrittliche PPH-Behandlungsgeräte landesweit weit verbreitet sind.
- In Deutschland besteht ein erheblicher Anteil der Bevölkerung, der von Erkrankungen betroffen ist, die mit schwerer PPH in Zusammenhang stehen, wie Mehrlingsschwangerschaften, Uterusmyomen, Anämie und dem HELLP-Syndrom. Dieses erhöhte Risiko treibt die Nachfrage nach wirksamen PPH-Behandlungslösungen voran und positioniert Deutschland als einen führenden Markt in Europa.
- In Deutschland sind wichtige Akteure im Bereich der PPH-Behandlungsgeräte ansässig, darunter Cook Medical und Utah Medical Products. Diese Unternehmen sind auf dem deutschen Markt stark vertreten und bieten eine Reihe innovativer Produkte wie Uterusballontamponaden an, die aufgrund ihrer einfachen Anwendung und Wirksamkeit sehr gefragt sind.
Deutschland wird voraussichtlich die höchste durchschnittliche jährliche Wachstumsrate auf dem Markt verzeichnen .“
- In Deutschland besteht ein erhebliches Risiko für Erkrankungen, die mit schwerer PPH in Zusammenhang stehen, wie Mehrlingsschwangerschaften, Uterusmyome, Anämie und das HELLP-Syndrom. Dieses erhöhte Risiko treibt die Nachfrage nach wirksamen PPH-Behandlungslösungen voran und positioniert Deutschland als einen führenden Markt in Europa.
- Deutschland verfügt über ein gut etabliertes Gesundheitssystem mit hohen Standards in der Mütterversorgung. Die proaktive Politik der Regierung und die erheblichen Investitionen in die Müttergesundheit tragen dazu bei, dass fortschrittliche PPH-Behandlungsgeräte landesweit weit verbreitet sind.
Marktanteil von Geräten zur Behandlung postpartaler Blutungen
Die Wettbewerbslandschaft des Marktes liefert detaillierte Informationen zu den einzelnen Wettbewerbern. Zu den Details gehören Unternehmensübersicht, Finanzzahlen, Umsatz, Marktpotenzial, Investitionen in Forschung und Entwicklung, neue Marktinitiativen, Präsenz in Europa, Produktionsstandorte und -anlagen, Produktionskapazitäten, Stärken und Schwächen des Unternehmens, Produkteinführung, Produktbreite und -umfang sowie Anwendungsdominanz. Die oben genannten Datenpunkte beziehen sich ausschließlich auf die Marktausrichtung der Unternehmen.
Die wichtigsten Marktführer auf dem Markt sind:
- BD (USA)
- Organon-Unternehmensgruppe (Niederlande)
- Laborie (USA)
- Cooper Companies (USA)
- Belmont Medical Technologies (USA)
- Utah Medical Products, Inc. (USA)
- Angiplast Private Limited (Indien)
- Krishco Medical Products Pvt. Ltd. (Indien)
- 3rd Stone Design (USA)
- Advin Health Care (USA)
- Coagulant Therapeutics Corporation (USA)
- Sterimed Group (USA)
- RevMedx (USA)
- Maternova Inc (USA)
- Sinapi Biomedical (USA)
Neueste Entwicklungen bei Geräten zur Behandlung postpartaler Blutungen
- Im April 2025 erwarb Organon von Biogen die US-Rechte an TOFIDENCE, einem Tocilizumab-Biosimilar zu ACTEMRA. Dies stärkt Organons Biosimilar-Portfolio im Bereich Immunologie und erweitert die Behandlungsmöglichkeiten für Arthritis und COVID-19. TOFIDENCE, das im Mai 2024 auf den Markt kam, behandelt verschiedene entzündliche Erkrankungen und unterstützt das Wachstum von Organons Biosimilar-Geschäft mit erheblichem Marktpotenzial.
- Im November 2023 hat CooperCompanies ausgewählte Vermögenswerte von Cook Medical für 300 Millionen US-Dollar übernommen und damit sein Portfolio im Bereich Frauengesundheit und Chirurgie unter CooperSurgical erweitert. Die Transaktion umfasst Produkte wie den Bakri-Ballon und Doppler-Monitore. Die Übernahme dürfte Umsatz und Gewinn im Jahr 2024 steigern und stärkt Coopers europäische Position im Bereich Fruchtbarkeits- und gynäkologische Gesundheitsversorgung.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Inhaltsverzeichnis
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 MARKET END USER COVERAGE GRID
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTAL ANALYSIS
4.2 PORTERS FIVE FORCES ANALYSIS
4.3 INDUSTRY INSIGHTS
4.3.1 MICRO AND MACROECONOMIC FACTORS
4.3.2 PENETRATION AND GROWTH PROSPECT MAPPING
4.3.3 KEY PRICING STRATEGIES
4.4 COST ANALYSIS BREAKDOWN
4.5 TECHNOLOGY ROADMAP
4.6 VALUE CHAIN ANALYSIS
4.7 OPPORTUNITY MAP ANALYSIS
4.8 HEALTHCARE ECONOMY
4.9 REIMBURSEMENT FRAMEWORK
4.1 TARIFFS AND ITS IMPACT ON THE MARKET
4.10.1 DEFINITION AND IMPORTANCE OF TARIFFS IN THE HEALTHCARE SECTOR
4.10.2 EUROPE VS. REGIONAL TARIFF STRUCTURES
4.10.3 IMPACT OF TARIFFS ON HEALTHCARE COSTS AND ACCESSIBILITY
4.10.4 TARIFF REGULATIONS IN KEY MARKETS
4.10.4.1 MEDICARE/MEDICAID TARIFF POLICIES
4.10.4.2 CMS PRICING MODELS
4.10.4.3 OTHERS
4.10.5 TARIFFS ON MEDICAL DEVICES & EQUIPMENT
4.10.5.1 IMPORT/EXPORT DUTIES ON MEDICAL EQUIPMENT
4.10.5.2 IMPACT ON PRICING AND AVAILABILITY OF HIGH-END MEDICAL TECHNOLOGY
4.10.5.3 CASE STUDIES OF TARIFF CHANGES AFFECTING THE INDUSTRY
4.10.6 COST BURDEN ON HOSPITALS AND HEALTHCARE FACILITIES
4.10.7 TARIFF EXEMPTIONS AND INCENTIVES
4.10.8 DUTY-FREE IMPORTS FOR ESSENTIAL MEDICINES AND VACCINES
4.10.9 IMPACT OF TRADE WARS ON THE HEALTHCARE SUPPLY CHAIN
4.10.10 ROLE OF FREE TRADE AGREEMENTS (FTAS) IN REDUCING TARIFFS
5 REGULATORY FRAMEWORK
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING INCIDENCE OF POSTPARTUM HAEMORRHAGE
6.1.2 ONGOING TECHNOLOGICAL ADVANCEMENTS FOR POSTPARTUM HEMORRHAGE TREATMENTS
6.1.3 RISING BIRTH RATES ASSOCIATED WITH AN INCREASE IN THE NUMBER OF POSTPARTUM HEMORRHAGE
6.1.4 REGULATORY SUPPORT AND APPROVALS ASSOCIATED WITH THE TREATMENT DEVICES
6.2 RESTRAINTS
6.2.1 SIDE EFFECTS ASSOCIATED WITH THE POSTPARTUM HEMORRHAGE TREATMENT
6.2.2 LIMITED RESEARCH AND DEVELOPMENT FOR PPH TREATMENT
6.3 OPPORTUNITIES
6.3.1 TRAINING AND EDUCATIONAL PROGRAMS FOR PROPER USE OF PPH TREATMENT DEVICES
6.3.2 SUPPORT FROM GOVERNMENTAL AND NON-GOVERNMENTAL ORGANIZATIONS IN PPH DEVICE ADOPTION
6.3.3 TELEMEDICINE INTEGRATION TO ENHANCE POSTPARTUM HEMORRHAGE DEVICE USE
6.4 CHALLENGES
6.4.1 ENVIRONMENTAL IMPACT AND DISPOSAL ISSUES OF SINGLE-USE PPH DEVICES
6.4.2 STERILITY CHALLENGES AND INFECTION RISKS IN PPH DEVICES
7 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE
7.1 OVERVIEW
7.2 UTERINE BALLOON TAMPONADE
7.2.1 BAKRI BALLOON
7.2.1.1 BAKRI POSTPARTUM BALLOON
7.2.1.2 BAKRI POSTPARTUM BALLOON WITH RAPID INSTILLATION COMPONENTS
7.2.2 FOLEY CATHETER
7.2.2.1 STANDARD FOLEY CATHETER
7.2.2.2 CONDOM-LOADED FOLEY CATHETER
7.3 UNIJECT PREFILLED INJECTION SYSTEM
7.3.1 OXYTOCIN-BASED INJECTION SYSTEM
7.3.2 CARBETOCIN-BASED INJECTION SYSTEM
7.4 NON-PNEUMATIC ANTI-SHOCK GARMENT
7.4.1 STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT
7.4.1.1 MEDIUM
7.4.1.2 LARGE
7.4.2 MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT
7.4.2.1 MEDIUM
7.4.2.2 LARGE
7.5 VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES
7.5.1 JADA SYSTEM
7.5.2 OTHERS
7.6 OTHERS
7.6.1 COMPRESSION DEVICES
7.6.1.1 B-LYNCH
7.6.1.2 HAYMAN
7.6.1.3 OTHER
7.6.2 UTERINE ARTERY LIGATION PRODUCTS
7.6.3 OTHERS
8 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE
8.1 OVERVIEW
8.2 PRIMARY PPH
8.3 SECONDARY PPH
9 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION
9.1 OVERVIEW
9.2 MAJOR POSTPARTUM HEMORRHAGE (MORE THAN 1000 ML)
9.3 MINOR POSTPARTUM HEMORRHAGE (500-1000 ML)
9.4 MASSIVE POSTPARTUM HEMORRHAGE (2000 ML OR MORE)
9.5 SECONDARY POSTPARTUM HEMORRHAGE
10 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL
10.1 OVERVIEW
10.2 DIRECT TENDER
10.3 RETAIL SALES
10.3.1 OFFLINE SALES
10.3.2 ONLINE SALES
10.4 OTHERS
11 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.2.1 PUBLIC HOSPITALS
11.2.1.1 TIER 2
11.2.1.2 TIER 3
11.2.1.3 TIER 1
11.2.2 PRIVATE HOSPITALS
11.2.2.1 TIER 2
11.2.2.2 TIER 3
11.2.2.3 TIER 1
11.3 MATERNITY CENTERS
11.4 SPECIALTY CLINICS
11.5 HOME CARE SETTINGS
11.6 OTHERS
12 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION
12.1 EUROPE
12.1.1 GERMANY
12.1.2 FRANCE
12.1.3 U.K.
12.1.4 ITALY
12.1.5 SPAIN
12.1.6 RUSSIA
12.1.7 TURKEY
12.1.8 NETHERLAND
12.1.9 POLAND
12.1.10 BELGIUM
12.1.11 SWITZERLAND
12.1.12 SWEDEN
12.1.13 DENMARK
12.1.14 NORWAY
12.1.15 FINLAND
12.1.16 REST OF EUROPE
13 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: GLOBAL
14 SWOT ANALYSIS
15 COMPANY PROFILES
15.1 BD
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 SOLUTION PORTFOLIO
15.1.5 RECENT NEWS
15.2 ORGANON GROUP OF COMPANIES
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENTS/NEWS
15.3 LABORIE
15.3.1 COMPANY SNAPSHOT
15.3.2 COMPANY SHARE ANALYSIS
15.3.3 PRODUCT PORTFOLIO
15.3.4 RECENT DEVELOPMENT
15.4 COOPERCOMPANIES
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENT
15.5 BELMONT MEDICAL TECHNOLOGIES
15.5.1 COMPANY SNAPSHOT
15.5.2 COMPANY SHARE ANALYSIS
15.5.3 PRODUCT PORTFOLIO
15.5.4 RECENT DEVELOPMENT
15.6 ADVIN HEALTH CARE
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENTS
15.7 ANGIPLAST PRIVATE LIMITED
15.7.1 COMPANY SNAPSHOT
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENT
15.8 COAGULANT THERAPEUTICS
15.8.1 COMPANY SNAPSHOT
15.8.2 PIPELINE PORTFOLIO
15.8.3 RECENT DEVELOPMENT
15.9 KRISHCO MEDICAL PRODUCTS PVT. LTD
15.9.1 COMPANY SNAPSHOT
15.9.2 PRODUCT PORTFOLIO
15.9.3 RECENT DEVELOPMENT
15.1 MATERNOVA INC.
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENT
15.11 REVMEDX
15.11.1 COMPANY SNAPSHOT
15.11.2 PRODUCT PORTFOLIO
15.11.3 RECENT DEVELOPMENT
15.12 3RD STONE DESIGN
15.12.1 COMPANY SNAPSHOT
15.12.2 PRODUCT PORTFOLIO
15.12.3 RECENT DEVELOPMENTS
15.13 STERIMED GROUP
15.13.1 COMPANY SNAPSHOT
15.13.2 PRODUCT PORTFOLIO
15.13.3 RECENT DEVELOPMENTS
15.14 UTAH MEDICAL PRODUCTS, INC.
15.14.1 COMPANY SNAPSHOT
15.14.2 REVENUE ANALYSIS
15.14.3 PRODUCT PORTFOLIO
15.14.4 RECENT DEVELOPMENTS
15.15 SINAPI BIOMEDICAL
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENTS
16 QUESTIONNAIRE
Tabellenverzeichnis
TABLE 1 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 2 EUROPE UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 3 EUROPE UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 4 EUROPE BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 5 EUROPE FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 6 EUROPE UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 7 EUROPE UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 8 EUROPE NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 9 EUROPE NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 10 EUROPE STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 11 EUROPE MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 12 EUROPE VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 13 EUROPE VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 14 EUROPE OTHERS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 15 EUROPE OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 16 EUROPE COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 17 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 18 EUROPE PRIMARY PPH IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 19 EUROPE SECONDARY PPH IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 20 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 21 EUROPE MAJOR POSTPARTUM HEMORRHAGE (MORE THAN 1000 ML) IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 22 EUROPE MINOR POSTPARTUM HEMORRHAGE (500-1000 ML) IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 23 EUROPE MASSIVE POSTPARTUM HEMORRHAGE (2000 ML OR MORE) IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 24 EUROPE SECONDARY POSTPARTUM HEMORRHAGE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 25 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 26 EUROPE DIRECT TENDER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 27 EUROPE RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 28 EUROPE RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 29 EUROPE OTHERS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 30 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 31 EUROPE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 32 EUROPE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 33 EUROPE PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 34 EUROPE PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 35 EUROPE MATERNITY CENTERS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 36 EUROPE SPECIALTY CLINICS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 37 EUROPE HOME CARE SETTINGS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 38 EUROPE OTHERS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 39 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 40 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 41 EUROPE UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 42 EUROPE BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 43 EUROPE FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 44 EUROPE UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 45 EUROPE NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 46 EUROPE STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 47 EUROPE MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 48 EUROPE VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 49 EUROPE OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 50 EUROPE COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 51 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 52 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 53 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 54 EUROPE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 55 EUROPE PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 56 EUROPE PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 57 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 58 EUROPE RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 59 GERMANY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 60 GERMANY UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 61 GERMANY BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 62 GERMANY FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 63 GERMANY UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 64 GERMANY NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 65 GERMANY STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 66 GERMANY MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 67 GERMANY VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 68 GERMANY OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 69 GERMANY COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 70 GERMANY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 71 GERMANY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 72 GERMANY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 73 GERMANY HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 74 GERMANY PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 75 GERMANY PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 76 GERMANY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 77 GERMANY RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 78 FRANCE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 79 FRANCE UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 80 FRANCE BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 81 FRANCE FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 82 FRANCE UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 83 FRANCE NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 84 FRANCE STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 85 FRANCE MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 86 FRANCE VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 87 FRANCE OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 88 FRANCE COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 89 FRANCE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 90 FRANCE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 91 FRANCE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 92 FRANCE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 93 FRANCE PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 94 FRANCE PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 95 FRANCE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 96 FRANCE RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 97 U.K. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 98 U.K. UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 99 U.K. BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 100 U.K. FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 101 U.K. UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 102 U.K. NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 103 U.K. STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 104 U.K. MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 105 U.K. VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 106 U.K. OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 107 U.K. COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 108 U.K. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 109 U.K. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 110 U.K. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 111 U.K. HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 112 U.K. PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 113 U.K. PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 114 U.K. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 115 U.K. RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 116 ITALY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 117 ITALY UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 118 ITALY BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 119 ITALY FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 120 ITALY UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 121 ITALY NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 122 ITALY STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 123 ITALY MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 124 ITALY VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 125 ITALY OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 126 ITALY COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 127 ITALY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 128 ITALY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 129 ITALY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 130 ITALY HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 131 ITALY PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 132 ITALY PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 133 ITALY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 134 ITALY RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 135 SPAIN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 136 SPAIN UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 137 SPAIN BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 138 SPAIN FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 139 SPAIN UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 140 SPAIN NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 141 SPAIN STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 142 SPAIN MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 143 SPAIN VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 144 SPAIN OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 145 SPAIN COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 146 SPAIN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 147 SPAIN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 148 SPAIN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 149 SPAIN HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 150 SPAIN PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 151 SPAIN PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 152 SPAIN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 153 SPAIN RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 154 RUSSIA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 155 RUSSIA UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 156 RUSSIA BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 157 RUSSIA FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 158 RUSSIA UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 159 RUSSIA NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 160 RUSSIA STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 161 RUSSIA MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 162 RUSSIA VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 163 RUSSIA OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 164 RUSSIA COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 165 RUSSIA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 166 RUSSIA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 167 RUSSIA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 168 RUSSIA HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 169 RUSSIA PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 170 RUSSIA PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 171 RUSSIA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 172 RUSSIA RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 173 TURKEY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 174 TURKEY UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 175 TURKEY BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 176 TURKEY FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 177 TURKEY UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 178 TURKEY NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 179 TURKEY STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 180 TURKEY MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 181 TURKEY VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 182 TURKEY OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 183 TURKEY COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 184 TURKEY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 185 TURKEY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 186 TURKEY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 187 TURKEY HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 188 TURKEY PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 189 TURKEY PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 190 TURKEY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 191 TURKEY RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 192 NETHERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 193 NETHERLAND UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 194 NETHERLAND BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 195 NETHERLAND FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 196 NETHERLAND UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 197 NETHERLAND NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 198 NETHERLAND STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 199 NETHERLAND MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 200 NETHERLAND VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 201 NETHERLAND OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 202 NETHERLAND COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 203 NETHERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 204 NETHERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 205 NETHERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 206 NETHERLAND HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 207 NETHERLAND PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 208 NETHERLAND PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 209 NETHERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 210 NETHERLAND RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 211 POLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 212 POLAND UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 213 POLAND BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 214 POLAND FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 215 POLAND UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 216 POLAND NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 217 POLAND STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 218 POLAND MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 219 POLAND VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 220 POLAND OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 221 POLAND COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 222 POLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 223 POLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 224 POLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 225 POLAND HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 226 POLAND PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 227 POLAND PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 228 POLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 229 POLAND RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 230 BELGIUM POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 231 BELGIUM UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 232 BELGIUM BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 233 BELGIUM FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 234 BELGIUM UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 235 BELGIUM NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 236 BELGIUM STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 237 BELGIUM MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 238 BELGIUM VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 239 BELGIUM OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 240 BELGIUM COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 241 BELGIUM POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 242 BELGIUM POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 243 BELGIUM POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 244 BELGIUM HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 245 BELGIUM PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 246 BELGIUM PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 247 BELGIUM POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 248 BELGIUM RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 249 SWITZERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 250 SWITZERLAND UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 251 SWITZERLAND BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 252 SWITZERLAND FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 253 SWITZERLAND UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 254 SWITZERLAND NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 255 SWITZERLAND STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 256 SWITZERLAND MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 257 SWITZERLAND VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 258 SWITZERLAND OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 259 SWITZERLAND COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 260 SWITZERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 261 SWITZERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 262 SWITZERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 263 SWITZERLAND HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 264 SWITZERLAND PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 265 SWITZERLAND PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 266 SWITZERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 267 SWITZERLAND RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 268 SWEDEN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 269 SWEDEN UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 270 SWEDEN BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 271 SWEDEN FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 272 SWEDEN UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 273 SWEDEN NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 274 SWEDEN STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 275 SWEDEN MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 276 SWEDEN VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 277 SWEDEN OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 278 SWEDEN COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 279 SWEDEN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 280 SWEDEN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 281 SWEDEN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 282 SWEDEN HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 283 SWEDEN PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 284 SWEDEN PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 285 SWEDEN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 286 SWEDEN RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 287 DENMARK POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 288 DENMARK UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 289 DENMARK BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 290 DENMARK FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 291 DENMARK UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 292 DENMARK NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 293 DENMARK STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 294 DENMARK MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 295 DENMARK VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 296 DENMARK OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 297 DENMARK COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 298 DENMARK POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 299 DENMARK POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 300 DENMARK POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 301 DENMARK HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 302 DENMARK PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 303 DENMARK PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 304 DENMARK POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 305 DENMARK RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 306 NORWAY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 307 NORWAY UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 308 NORWAY BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 309 NORWAY FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 310 NORWAY UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 311 NORWAY NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 312 NORWAY STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 313 NORWAY MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 314 NORWAY VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 315 NORWAY OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 316 NORWAY COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 317 NORWAY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 318 NORWAY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 319 NORWAY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 320 NORWAY HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 321 NORWAY PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 322 NORWAY PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 323 NORWAY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 324 NORWAY RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 325 FINLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 326 FINLAND UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 327 FINLAND BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 328 FINLAND FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 329 FINLAND UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 330 FINLAND NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 331 FINLAND STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 332 FINLAND MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 333 FINLAND VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 334 FINLAND OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 335 FINLAND COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 336 FINLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 337 FINLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 338 FINLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 339 FINLAND HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 340 FINLAND PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 341 FINLAND PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 342 FINLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 343 FINLAND RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 344 REST OF EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
Abbildungsverzeichnis
FIGURE 1 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: SEGMENTATION
FIGURE 2 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: DROC ANALYSIS
FIGURE 4 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: VENDOR SHARE ANALYSIS
FIGURE 9 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: MARKET END USER COVERAGE GRID
FIGURE 10 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: SEGMENTATION
FIGURE 11 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: EXECUTIVE SUMMARY
FIGURE 12 STRATEGIC DECISIONS
FIGURE 13 RISING INCIDENCE OF POSTPARTUM HAEMORRHAGE IS EXPECTED TO DRIVE THE EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 14 UTERINE BALLOON TAMPONADE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET IN THE FORECAST PERIOD OF 2025 & 2032
FIGURE 15 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE (2024)
FIGURE 16 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET
FIGURE 17 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY TYPE, 2024
FIGURE 18 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY TYPE, 2025-2032 (USD THOUSAND)
FIGURE 19 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY TYPE, CAGR (2025-2032)
FIGURE 20 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY TYPE, LIFELINE CURVE
FIGURE 21 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY PATIENT TYPE, 2024
FIGURE 22 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY PATIENT TYPE, 2025-2032 (USD THOUSAND)
FIGURE 23 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY PATIENT TYPE, CAGR (2025-2032)
FIGURE 24 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY PATIENT TYPE, LIFELINE CURVE
FIGURE 25 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY CONDITION, 2024
FIGURE 26 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY CONDITION, 2025-2032 (USD THOUSAND)
FIGURE 27 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY CONDITION, CAGR (2025-2032)
FIGURE 28 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY CONDITION, LIFELINE CURVE
FIGURE 29 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 30 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY DISTRIBUTION CHANNEL, 2025-2032 (USD THOUSAND)
FIGURE 31 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025-2032)
FIGURE 32 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 33 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY END USER, 2024
FIGURE 34 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY END USER, 2025-2032 (USD THOUSAND)
FIGURE 35 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY END USER, CAGR (2025-2032)
FIGURE 36 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY END USER, LIFELINE CURVE
FIGURE 37 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: SNAPSHOT (2024)
FIGURE 38 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: COMPANY SHARE 2024 (%)

Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.