Middle East And Africa Point Care Testing Poct Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
3.15 Billion
USD
7.11 Billion
2025
2033
USD
3.15 Billion
USD
7.11 Billion
2025
2033
| 2026 –2033 | |
| USD 3.15 Billion | |
| USD 7.11 Billion | |
|
|
|
|
Markt für patientennahe Sofortdiagnostik (POCT) im Nahen Osten und Afrika, nach Produkttyp (Glukosemonitoringprodukte, Produkte für Infektionskrankheitstests, Produkte für kardiometabolische Überwachung, Produkte für Schwangerschafts- und Fruchtbarkeitstests, Produkte für hämatologische Tests, Produkte für Gerinnungsmonitoring, Produkte für Drogenmissbrauchstests, Produkte für Urinanalysen, Produkte für Cholesterintests, Produkte für Tumor-/Krebsmarkertests, Produkte für Stuhltests auf okkulte Substanzen und Sonstige), nach Plattform (Lateral-Flow-Assays/Immunochromatographie-Tests, Immunoassays, Teststreifen, Molekulardiagnostik, klinisch-chemische Assays, Mikrofluidik, Hämatologie und Sonstige), nach Anwendung (Blutzucker, Infektionskrankheiten, Vitalparameter-Überwachung, Herzüberwachung, Gerinnung, Hämatologie, nicht-invasive SpO2-Überwachung, Bluttransfusion, nicht-invasive pCO2-Überwachung, Vollblutanalyse und Sonstige) und nach Verschreibungsmodus (verschreibungspflichtige Tests und rezeptfreie Tests). Gegentests, nach Endnutzer (Krankenhäuser, häusliche Pflege, Kliniken, Labore, Diagnosezentren, Pathologielabore, ambulante Operationszentren, Altenpflegeeinrichtungen, Sonstige), nach Vertriebskanal (Direktvergabe, Einzelhandel, Online-Handel, Sonstige), Land (Südafrika, Saudi-Arabien, Vereinigte Arabische Emirate, Ägypten, Israel, Bahrain, Kuwait, Oman, Katar, übriger Naher Osten und Afrika) – Branchentrends und Prognose bis 2033
Marktgröße für patientennahe Sofortdiagnostik (POCT) im Nahen Osten und Afrika
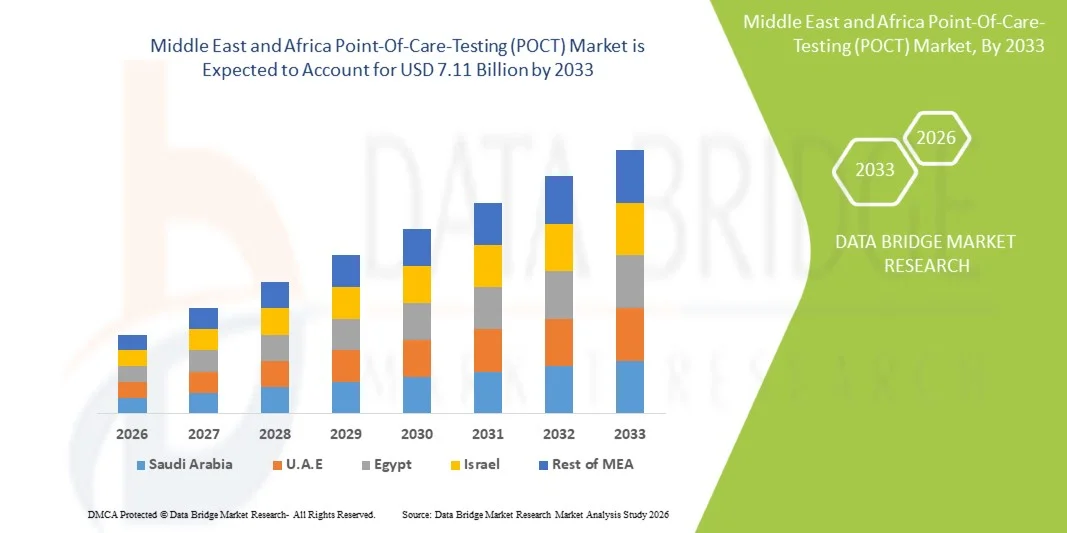
- Der Markt für patientennahe Sofortdiagnostik (POCT) im Nahen Osten und Afrika hatte im Jahr 2025 einen Wert von 3,15 Milliarden US-Dollar und wird voraussichtlich bis 2033 auf 7,11 Milliarden US-Dollar anwachsen , was einer durchschnittlichen jährlichen Wachstumsrate (CAGR) von 10,8 % im Prognosezeitraum entspricht.
- Das Wachstum des Marktes für patientennahe Sofortdiagnostik (POCT) im Nahen Osten und in Afrika wird primär durch die steigende Nachfrage nach schnellen Diagnoselösungen getrieben, die eine sofortige klinische Entscheidungsfindung ermöglichen und die Abhängigkeit von zentralisierten Labortests verringern. POCT-Geräte ermöglichen kürzere Bearbeitungszeiten, eine frühzeitige Krankheitserkennung und verbesserte Patientenergebnisse, was insbesondere in der Notfallversorgung, der Telemedizin und der häuslichen Überwachung von entscheidender Bedeutung ist.
- Die zunehmende Verbreitung chronischer Erkrankungen wie Diabetes, Herz-Kreislauf-Erkrankungen, Infektionskrankheiten und Atemwegserkrankungen führt zu einer deutlich steigenden Nachfrage nach Point-of-Care-Testgeräten (POCT) für die Echtzeitüberwachung und -behandlung. Fortschritte in der Diagnostik, der Mikrofluidik, bei Biosensoren und vernetzten Gesundheitsplattformen verbessern zudem Genauigkeit, Mobilität und Datenintegrationsmöglichkeiten und treiben so das Marktwachstum an.
Marktanalyse für patientennahe Sofortdiagnostik (POCT) im Nahen Osten und Afrika
- Der Markt für patientennahe Sofortdiagnostik (Point-of-Care-Testing, POCT) im Nahen Osten und in Afrika ist ein schnell wachsendes Segment der Diagnostikbranche. Er bietet sofortige Testergebnisse direkt am Patientenstandort, beispielsweise in Kliniken, Krankenhäusern, Rettungswagen oder zu Hause. POCT-Geräte ermöglichen ein schnelles Screening und Monitoring von Erkrankungen wie Diabetes, Infektionskrankheiten, Schwangerschaft, Herzmarkern, Elektrolytstörungen und Gerinnungsparametern. Dies trägt zu einer höheren klinischen Effizienz und einer Entlastung des Gesundheitssystems bei.
- Die steigende Nachfrage nach tragbaren und benutzerfreundlichen Diagnosetechnologien wird maßgeblich durch die zunehmende Verbreitung chronischer Erkrankungen, den Bedarf an Notfallversorgung und die Ausweitung der häuslichen Patientenüberwachung angetrieben. Darüber hinaus trägt die Integration digitaler Gesundheitsplattformen, smartphonebasierter Diagnostik, KI-gestützter Erkennung und drahtloser Konnektivität zu einer verbesserten Datennachverfolgbarkeit und Fernbehandlung von Patienten bei und fördert so das Marktwachstum zusätzlich.
- Saudi-Arabien wird voraussichtlich den Markt für patientennahe Sofortdiagnostik (POCT) im Nahen Osten und in Afrika dominieren und im Jahr 2026 mit einem Umsatzanteil von rund 30,00 % den größten Marktanteil halten. Dies ist auf die starke Präsenz wichtiger Marktteilnehmer, hohe Gesundheitsausgaben, eine fortschrittliche medizinische Infrastruktur und die zunehmende Nutzung innovativer Diagnosetechnologien in Krankenhäusern und der häuslichen Pflege zurückzuführen.
- Es wird erwartet, dass das Segment der Glukoseüberwachungsprodukte den POCT-Markt im Nahen Osten und in Afrika mit einem bedeutenden Anteil von über 43,07 % im Jahr 2026 dominieren wird. Treiber dieser Entwicklung sind die steigende Zahl von Diabetikern, die Präferenz für Echtzeit-Überwachungsgeräte und die zunehmende Verwendung von tragbaren Blutzuckermessgeräten und kontinuierlichen Glukoseüberwachungssystemen bei Patienten in der häuslichen Pflege.
Berichtsgegenstand und Marktsegmentierung für patientennahe Sofortdiagnostik (POCT) im Nahen Osten und Afrika
|
Attribute |
Point-of-Care-Testing (POCT) im Nahen Osten und Afrika: Wichtigste Markteinblicke |
|
Abgedeckte Segmente |
|
|
Abgedeckte Länder |
Naher Osten und Afrika
|
|
Wichtige Marktteilnehmer |
|
|
Marktchancen |
|
|
Mehrwertdaten-Infosets |
Zusätzlich zu Einblicken in Marktszenarien wie Marktwert, Wachstumsrate, Segmentierung, geografische Abdeckung und Hauptakteure enthalten die von Data Bridge Market Research erstellten Marktberichte auch einen Innovationstracker und strategische Analysen, technologische Fortschritte, Klimawandelszenarien, Lieferkettenanalysen, Wertschöpfungskettenanalysen, Kriterien für die Anbieterauswahl, PESTLE-Analysen, Porter-Analysen, Patentanalysen, Analysen des Branchenökosystems, Rohstoffabdeckung, Zölle und deren Auswirkungen auf den Markt, Regulierungsabdeckung, Kaufverhalten der Verbraucher, Markenaussichten, Kostenanalyseaufschlüsselung und regulatorische Rahmenbedingungen. |
Markttrends für patientennahe Sofortdiagnostik (POCT) im Nahen Osten und Afrika
„Technologische Fortschritte und funktionale Erweiterung durch Forschung und Entwicklung sowie digitale Integration “
- Ein wichtiger und sich rasant entwickelnder Trend im Markt für patientennahe Sofortdiagnostik (POCT) im Nahen Osten und in Afrika ist der zunehmende Fokus auf Innovation, Forschung und Entwicklung sowie fortschrittliche Diagnosetechnologien mit dem Ziel, Genauigkeit, Geschwindigkeit, Mobilität und Echtzeit-Entscheidungsfindung zu verbessern. Angesichts der steigenden Nachfrage nach dezentralen und patientenzentrierten Tests investieren Gesundheitsdienstleister, Diagnostikunternehmen und Medizintechnikhersteller verstärkt in POCT-Plattformen der nächsten Generation, die präzise Diagnostik und verbesserte Vernetzung in verschiedenen Behandlungseinrichtungen ermöglichen.
- Führende Unternehmen wie Abbott, Roche, Siemens Healthineers, Danaher und Thermo Fisher Scientific intensivieren ihre Forschungsbemühungen zur Entwicklung miniaturisierter Geräte, Lab-on-a-Chip-Technologien und KI-gestützter Testsysteme, die Laborergebnisse direkt vor Ort liefern. Zu diesen technologischen Fortschritten zählen auch die automatisierte Probenverarbeitung, eine verbesserte Biosensor-Empfindlichkeit und Multiplex-Testverfahren, die die gleichzeitige Detektion mehrerer Biomarker ermöglichen.
- In der Infektionsdiagnostik konzentrieren sich umfangreiche Forschungs- und Entwicklungsinitiativen auf die Entwicklung schneller molekularer Testlösungen mit hoher Genauigkeit, kurzen Bearbeitungszeiten und Eignung für Notfall- und Ausbruchssituationen. Point-of-Care-Testgeräte (POCT) für Influenza, COVID-19, Atemwegsinfektionen, HIV und Sepsis werden durch Mikrofluidik und isotherme Amplifikationstechnologien verbessert und ermöglichen so zuverlässige Tests außerhalb zentralisierter Labore.
- Im chronischen Krankheitsmanagement unterstützen Innovationen bei der Glukosemessung, der Bestimmung von Herzbiomarkern, der Gerinnungsanalyse und der Nierenfunktionsanalyse die kontinuierliche Überwachung und liefern Echtzeitdaten für klinische Interventionen. Unternehmen integrieren mobile Anwendungen, cloudbasierte Berichtssysteme und tragbare Diagnostik, um eine nahtlose Überwachung zu Hause und telemedizinische Fernbehandlung zu ermöglichen.
- Der Markt verzeichnet zudem ein Wachstum im Bereich der personalisierten Diagnostik. Point-of-Care-Testlösungen (POCT) werden entwickelt, um die Onkologie, Reproduktionsmedizin, Magen-Darm-Erkrankungen und das Screening von Stoffwechselerkrankungen zu unterstützen. Diese Innovationen zielen darauf ab, eine frühere Erkennung, schnellere Therapieentscheidungen und verbesserte klinische Ergebnisse zu ermöglichen, insbesondere im ambulanten und häuslichen Bereich.
- Darüber hinaus ermöglicht die Integration vernetzter Geräte, digitaler Datenanalyse und KI-gestützter Interpretation intelligentere POCT-Ökosysteme, die die Workflow-Automatisierung verbessern und menschliche Fehler reduzieren. Diese Fortschritte wandeln POCT in ein multifunktionales und intelligentes Diagnosewerkzeug um, das Prävention, Präzisionsmedizin und Bevölkerungsgesundheitsmanagement unterstützt.
- Dieses sich rasant entwickelnde, innovationsgetriebene Umfeld prägt den POCT-Markt neu und verschiebt die Branche hin zu tragbaren, integrierten und patientenzentrierten Diagnosesystemen. Da die Gesundheitssysteme im Nahen Osten und in Afrika Effizienz, Zugänglichkeit und Nachhaltigkeit priorisieren, wird erwartet, dass die forschungsgetriebene digitale Transformation neue Anwendungen erschließt und die Marktdurchdringung in entwickelten und aufstrebenden Regionen ausweitet.
Marktdynamik für patientennahe Sofortdiagnostik (POCT) im Nahen Osten und Afrika
Treiber
„Steigende Nachfrage nach schnellen, dezentralen und patientenorientierten Diagnoselösungen“
- Ein wesentlicher Wachstumstreiber des Marktes für patientennahe Sofortdiagnostik (POCT) im Nahen Osten und in Afrika ist der steigende Bedarf an schnellen, leicht zugänglichen und dezentralen Diagnoseverfahren, die die klinische Entscheidungsfindung und die Behandlungsergebnisse verbessern. Im Zuge der Umstellung der Gesundheitssysteme auf wertorientierte und patientenzentrierte Modelle liefert POCT sofortige Testergebnisse direkt am Behandlungsort oder in dessen unmittelbarer Nähe. Dadurch wird die Abhängigkeit von zentralen Laboren reduziert und eine frühzeitige Diagnose sowie ein rechtzeitiger Behandlungsbeginn ermöglicht.
- Führende Unternehmen der Branche wie Abbott, Roche, Siemens Healthineers und Danaher investieren verstärkt in Forschung und Entwicklung, um tragbare, hochpräzise und digital vernetzte Point-of-Care-Testgeräte (POCT) zu entwickeln, die Echtzeitdiagnostik in Krankenhäusern, Kliniken, Notaufnahmen und der häuslichen Pflege ermöglichen. Diese Innovationen entsprechen der steigenden Nachfrage nach integrierten digitalen Gesundheitslösungen, dem Ausbau der Telemedizin und KI-gestützter Ergebnisinterpretation.
- Im Bereich des chronischen Krankheitsmanagements erleben Point-of-Care-Tests (POCT) zur Glukosemessung, Bestimmung von Herzmarkern, Gerinnungstests und Nierenfunktionsprüfungen ein rasantes Wachstum. Grund dafür ist die zunehmende Verbreitung von Diabetes, Herz-Kreislauf-Erkrankungen und Zivilisationskrankheiten im Nahen Osten und in Afrika. Ebenso werden POCT-Systeme für Infektionskrankheiten wie COVID-19, Grippe, Sepsis, Malaria und HIV zu unverzichtbaren Instrumenten der Ausbruchskontrolle, insbesondere in ressourcenarmen Regionen.
- Der zunehmende Fokus auf die Überwachung von Patienten zu Hause und aus der Ferne treibt die Akzeptanz ebenfalls voran, unterstützt durch die Entwicklung von Smartphone-gestützten Diagnoseverfahren, tragbaren Testlösungen, Cloud-basierten Plattformen und Fernmeldefunktionen, die die klinische Vernetzung und die Kontinuität der Versorgung verbessern.
- Mit der Modernisierung der Gesundheitsinfrastruktur, dem zunehmenden Fokus auf schnelle Diagnoseergebnisse und starken öffentlichen und privaten Investitionen entwickelt sich die patientennahe Labordiagnostik (POCT) zu einem unverzichtbaren Bestandteil der zukünftigen Gesundheitsversorgung. Da Effizienz und Zugänglichkeit im Zentrum der Gesundheitsreform im Nahen Osten und in Afrika stehen, wird die Nachfrage nach innovativen, präzisen und patientenorientierten POCT-Systemen voraussichtlich deutlich steigen.
Zurückhaltung/Herausforderung
„ Hohe Kosten, regulatorische Komplexität sowie Bedenken hinsichtlich Genauigkeit und Standardisierung “
- Trotz starken Marktwachstums steht der Markt für patientennahe Sofortdiagnostik (POCT) im Nahen Osten und Afrika vor erheblichen Herausforderungen. Diese betreffen hohe Geräte- und Testkosten, strenge Zulassungsverfahren und die schwankende diagnostische Genauigkeit verschiedener Geräteplattformen. Die Gewährleistung einer gleichbleibenden analytischen Leistung, vergleichbar mit den Standards zentralisierter Labore, bleibt eine zentrale Hürde, insbesondere mit der zunehmenden Verbreitung von POCT in der Intensivmedizin und bei hochkomplexen Tests.
- Die Zulassung durch Aufsichtsbehörden wie die FDA, die EMA und andere nationale Behörden erfordert umfangreiche klinische Validierung, die Einhaltung von Qualitätsstandards und die Überwachung nach der Markteinführung. Diese Prozesse können die Markteinführungszeiten erheblich verlängern und die Entwicklungskosten für Hersteller erhöhen, insbesondere bei neuen Diagnosetechnologien wie molekularen Point-of-Care-Tests (POCT) und Multiplex-Tests.
- Kostenbeschränkungen hemmen die Einführung auch in Entwicklungsländern, wo begrenzte Gesundheitsbudgets und unzureichende Erstattungssysteme eine flächendeckende Implementierung verhindern. Darüber hinaus können betriebliche Herausforderungen wie Wartungsaufwand, Schulungsbedarf für Anwender und die Integration in Arbeitsabläufe eine großflächige Einführung in Krankenhäusern und Diagnosezentren behindern.
- Bedenken hinsichtlich der Genauigkeit, Zuverlässigkeit und Standardisierung von Ergebnissen – insbesondere bei hochsensitiven Tests auf Infektionskrankheiten und Herzmarker – können bei Ärzten zu Unsicherheit führen und die Abhängigkeit von zentralisierten Bestätigungstests erhöhen. Probleme im Zusammenhang mit der Datenintegration und Cybersicherheit digitaler Point-of-Care-Testplattformen erfordern zudem verbesserte technologische Rahmenbedingungen und Investitionen.
- Mit der Weiterentwicklung der Point-of-Care-Testtechnologie (POCT) werden zur Bewältigung dieser finanziellen, regulatorischen und technischen Herausforderungen umfangreiche Forschungs- und Entwicklungsinvestitionen, eine verbesserte Harmonisierung der Diagnosestandards im Nahen Osten und in Afrika sowie eine strategische Zusammenarbeit zwischen Geräteherstellern, Gesundheitsdienstleistern und staatlichen Gesundheitsbehörden erforderlich sein. Bis dahin könnten diese Hürden die Einführung in bestimmten Marktsegmenten und Regionen weiterhin verlangsamen.
Marktübersicht für patientennahe Sofortdiagnostik (POCT) im Nahen Osten und Afrika
Der Markt ist segmentiert nach Produkttyp, Plattform, Anwendung, Verschreibungsmodus, Endnutzer und Vertriebskanal.
- Nach Produkttyp
Basierend auf dem Produkttyp ist der Markt für patientennahe Sofortdiagnostik (POCT) im Nahen Osten und Afrika in folgende Segmente unterteilt: Produkte zur Blutzuckermessung, Produkte zur Infektionsdiagnostik, Produkte zur kardiometabolischen Überwachung, Produkte für Schwangerschafts- und Fruchtbarkeitstests, Produkte für hämatologische Tests, Produkte zur Gerinnungsüberwachung, Produkte für Drogentests, Urinanalysen, Cholesterintests, Produkte für Tumor-/Krebsmarkertests, Produkte für Stuhltests auf okkulte Keime und Sonstiges. Das Segment der Produkte zur Blutzuckermessung wird voraussichtlich im Jahr 2026 mit einem Marktanteil von 43,07 % den größten Umsatz erzielen. Treiber dieser Entwicklung sind die zunehmende Diabetesprävalenz im Nahen Osten und Afrika, die steigende Nachfrage nach häuslicher Selbstkontrolle sowie die kontinuierlichen technologischen Fortschritte bei Blutzuckermessgeräten und vernetzten digitalen Gesundheitsplattformen.
- Nach Plattform
Basierend auf der Plattform ist der Markt für patientennahe Sofortdiagnostik (POCT) im Nahen Osten und Afrika in folgende Segmente unterteilt: Lateral-Flow-Tests/Immunochromatographie-Tests, Immunoassays, Schnelltests, Molekulardiagnostik, klinisch-chemische Tests, Mikrofluidik, Hämatologie und Sonstige. Lateral-Flow-Tests/Immunochromatographie-Tests werden voraussichtlich 2026 den Markt dominieren. Gründe hierfür sind ihre breite Anwendung im Screening auf Infektionskrankheiten, ihre einfache Handhabung, die schnelle Ergebnisbereitstellung, die geringen Kosten und ihr umfangreicher Einsatz bei Ausbrüchen wie COVID-19, Grippe, Malaria und Denguefieber. Ihre Eignung für dezentrale und abgelegene Gesundheitseinrichtungen stärkt das Wachstum dieses Segments zusätzlich.
- Durch Bewerbung
Basierend auf den Anwendungsbereichen ist der Markt für patientennahe Sofortdiagnostik (POCT) im Nahen Osten und Afrika in folgende Segmente unterteilt: Blutzucker, Infektionskrankheiten, Vitalparameter-Überwachung, Herzüberwachung, Gerinnung, Hämatologie, nicht-invasive SpO2-Messung, Bluttransfusion, nicht-invasive PCO2-Messung, Vollblutanalyse und Sonstiges. Es wird erwartet, dass der Bereich Blutzucker im Jahr 2026 mit dem größten Marktanteil führend sein wird. Treiber dieser Entwicklung sind die steigende Diabetesprävalenz, die zunehmende Präferenz der Patienten für tragbare Überwachungsgeräte sowie die Verfügbarkeit hochpräziser, benutzerfreundlicher Blutzuckermessgeräte und Technologien zur kontinuierlichen Glukosemessung (CGM), die ein Diabetesmanagement in Echtzeit ermöglichen.
- Nach Rezeptmodus
Basierend auf dem Verschreibungsmodell ist der Markt für patientennahe Sofortdiagnostik (POCT) im Nahen Osten und Afrika in rezeptfreie Tests und verschreibungspflichtige Tests unterteilt. Das Segment der rezeptfreien Tests erzielte 2026 den größten Marktanteil, bedingt durch die steigende Nachfrage der Verbraucher nach Selbsttests, die zunehmende Verfügbarkeit von Schnelltests und den wachsenden Fokus auf Prävention. Rezeptfreie POCT-Geräte ermöglichen es Anwendern, Gesundheitsparameter wie Blutzuckerwerte, Schwangerschaft, Fruchtbarkeit, Infektionskrankheiten und Herz-Kreislauf-Indikatoren ohne ärztliche Aufsicht zu überwachen.
- Vom Endbenutzer
Basierend auf den Endnutzern ist der Markt für patientennahe Sofortdiagnostik (POCT) im Nahen Osten und Afrika in Krankenhäuser, häusliche Pflege, Kliniken, Labore, Diagnosezentren, Pathologielabore, ambulante Operationszentren, Altenpflegeeinrichtungen und Sonstige unterteilt. Das Segment der Krankenhäuser erzielte 2026 den größten Marktanteil, getrieben durch die hohe Nachfrage nach schnellen Diagnosetests für Notfall-, Intensiv- und Routineuntersuchungen. POCT-Geräte – wie Blutzuckermessgeräte, Herzmarker-Analysegeräte, Schnelltests für Infektionskrankheiten, Gerinnungsmonitore und Blutgasanalysatoren – werden zunehmend in Krankenhäusern eingesetzt, um schnellere klinische Entscheidungen zu ermöglichen, die Wartezeiten für Patienten zu verkürzen und die Arbeitsabläufe effizienter zu gestalten.
- Nach Vertriebskanal
Basierend auf dem Vertriebskanal ist der Markt für patientennahe Sofortdiagnostik (POCT) im Nahen Osten und Afrika in Direktvergabe, Einzelhandel, Online-Handel und Sonstige unterteilt. Das Segment Direktvergabe erzielte 2026 den größten Marktanteil, bedingt durch die hohe Nachfrage nach POCT-Geräten durch Krankenhäuser, Diagnoselabore und Gesundheitsbehörden. Groß angelegte Ausschreibungen gewährleisten die kontinuierliche Versorgung mit essenziellen Diagnoseinstrumenten – wie Blutzuckermessgeräten, Schnelltests für Infektionskrankheiten, Herzbiomarker-Analysegeräten und Gerinnungsmessgeräten –, die für Routineuntersuchungen und Notfallversorgung benötigt werden.
Regionale Analyse des Point-of-Care-Testing-Marktes (POCT) im Nahen Osten und Afrika
- Der Nahe Osten und Afrika dominierten 2025 den Markt für patientennahe Sofortdiagnostik (POCT) mit einem Umsatzanteil von über 4,98 %. Treiber dieser Entwicklung waren die rasch wachsende Gesundheitsinfrastruktur der Region, die zunehmende Belastung durch chronische Krankheiten und die verstärkte Nutzung dezentraler Diagnosetechnologien. Länder wie die USA, Kanada und Mexiko verzeichnen eine starke Nachfrage nach Schnelltests für Diabetes, Infektionskrankheiten, Herz-Kreislauf-Erkrankungen und die häusliche Überwachung.
- Steigende Investitionen in digitale Gesundheit, der Ausbau der Telemedizin und staatliche Initiativen zur Früherkennung beschleunigen die Einführung von Point-of-Care-Tests (POCT) zusätzlich. Darüber hinaus verstärken die große Zahl älterer Menschen und die wachsende Nachfrage der Verbraucher nach kostengünstigen und schnellen medizinischen Tests das Marktwachstum in der gesamten Region.
Markteinblicke für patientennahe Sofortdiagnostik (POCT) in Saudi-Arabien, dem Nahen Osten und Afrika
Saudi-Arabien dominierte 2025 den POCT-Markt im Nahen Osten und in Afrika. Gründe hierfür waren die fortschrittliche Gesundheitsinfrastruktur, das hohe Bewusstsein für die Früherkennung von Krankheiten und die zunehmende Beliebtheit von Diagnoselösungen für zu Hause. Die steigende Verbreitung von Diabetes, Herzerkrankungen, Atemwegserkrankungen und Infektionskrankheiten treibt die Nachfrage nach Schnelltests weiter an. Starke Forschungs- und Entwicklungskapazitäten, die hohe Akzeptanz vernetzter und in Smartphones integrierter Testgeräte sowie ein ausgereiftes regulatorisches Umfeld tragen zusätzlich zum Marktwachstum bei. Darüber hinaus stärkt der Trend zu personalisierter und bedarfsgerechter Diagnostik – in Verbindung mit dem Anstieg von ambulanten Kliniken und apothekenbasierten Testangeboten – die POCT-Landschaft in Saudi-Arabien weiter.
Marktanteil von Point-of-Care-Tests (POCT) im Nahen Osten und Afrika
Die Point-of-Care-Testing-Branche (POCT) wird hauptsächlich von etablierten Unternehmen dominiert, darunter:
- Abbott Point of Care Inc. (USA)
- Sinocare Inc. (China)
- F. Hoffmann-La Roche AG (Schweiz)
- Danaher Corporation (USA)
- Hologic, Inc. (USA)
- bioMérieux SA (Frankreich)
- Siemens Healthineers AG (Deutschland)
- Thermo Fisher Scientific Inc. (USA)
- BD Veritor (Becton, Dickinson and Company) (USA)
- QuidelOrtho Corporation (USA)
- Bio-Rad Laboratories, Inc. (USA)
- Werfen (Spanien)
- Sekisui Diagnostics (Japan)
- Trividia Health, Inc. (USA)
- Nova Biomedical Corporation (USA)
- Meridian Bioscience, Inc. (USA)
- Pfizer Inc. (USA)
- Shenzhen New Industry Biomedical Engineering Co., Ltd. (China)
- Sysmex Corporation (Japan)
- Wondfo (Guangzhou Wondfo Biotech Co., Ltd.) (China)
- QIAGEN NV (Deutschland)
- Abaxis, Inc. (USA)
- Autobio Diagnostics Co., Ltd. (China)
- Getein Biotech, Inc. (China)
- Chembio Diagnostics, Inc. (USA)
- EKF Diagnostics Holdings plc (UK)
- Trinity Biotech plc (Irland)
- PTS Diagnostics (USA)
- QuantuMDx Group Ltd. (UK)
- Binx Health (USA)
- Xiamen Boson Biotech Co., Ltd. (China)
- Accubiotech Co., Ltd. (China)
- Sienco, Inc. (USA)
- LambdaGen Corporation (USA)
Neueste Entwicklungen auf dem Markt für patientennahe Sofortdiagnostik (POCT) im Nahen Osten und in Afrika
- Im Mai 2020 lieferte Abbotts ID NOW COVID-19-Test schnelle und zuverlässige Ergebnisse innerhalb weniger Minuten und trug so zu einer zeitnahen Diagnose und zur Reduzierung des Infektionsrisikos bei. Studien belegen seine hohe Leistungsfähigkeit in Notfallambulanzen mit einer Sensitivität von ≥ 94,7 % und einer Spezifität von ≥ 98,6 %. Trotz Kritikpunkten einer Studie der NYU bestätigen Daten aus der Praxis seine Wirksamkeit. Der Test, der im Rahmen der Notfallzulassung der FDA zugelassen wurde, spielt eine entscheidende Rolle bei der COVID-19-Diagnostik.
- Im September 2025 erhielt Dongguan E-Test Technology, eine Tochtergesellschaft von Sinocare, die FDA-Zulassung (510(k)) für ihre intelligenten Blutdruckmessgeräte der Multi-Serie. Die Geräte zeichnen sich durch Genauigkeit, Sicherheit und drahtlose Funktionalität aus. Sie bieten medizinische Überwachung, Bluetooth-Konnektivität und intelligente Warnmeldungen und stärken damit Sinocares Expansionsstrategie im Nahen Osten und in Afrika. Gleichzeitig erweitern sie ihr Angebot im Bereich des Managements chronischer Erkrankungen auf internationalen Märkten, darunter in den USA und Europa.
- Im Januar 2025 schloss die Danaher Corporation eine Investitionspartnerschaft mit Innovaccer Inc., einem Unternehmen für KI im Gesundheitswesen. Ziel dieser Zusammenarbeit ist es, die Einführung von Präzisionsdiagnostik und wertorientierter Versorgung zu beschleunigen, indem Gesundheitsdienstleistern einheitliche Patientendaten und fortschrittliche Analysen zur Verfügung gestellt werden. Dadurch sollen die Behandlungsergebnisse durch personalisierte und zeitnahe Interventionen verbessert werden.
- Im November 2023 ging Binx Health eine Partnerschaft mit Fisher Healthcare ein, um den Vertrieb des FDA-zugelassenen binx io, einer molekularen Point-of-Care-Plattform zum Nachweis von Chlamydien und Gonorrhö, auszuweiten. Das System liefert Ergebnisse in Laborqualität innerhalb von etwa 30 Minuten, verbessert so die zeitnahe Diagnosestellung und ermöglicht es Ärzten, Patienten im Rahmen eines einzigen Besuchs zu testen und zu behandeln, wodurch der Zugang zur Gesundheitsversorgung optimiert wird.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Inhaltsverzeichnis
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 MULTIVARIATE MODELLING
2.6 TYPE LIFELINE CURVE
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 MARKET END USER COVERAGE GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER’S FIVE FORCES
4.2 PESTEL ANALYSIS
4.3 HEALTHCARE ECONOMY
4.3.1 HEALTHCARE EXPENDITURE
4.3.2 CAPITAL EXPENDITURE
4.3.3 CAPEX TRENDS
4.3.4 CAPEX ALLOCATION
4.3.5 FUNDING SOURCES
4.3.6 INDUSTRY BENCHMARKS
4.3.7 GDP RATIO IN OVERALL GDP
4.3.8 HEALTHCARE SYSTEM STRUCTURE
4.3.9 GOVERNMENT POLICIES
4.3.10 ECONOMIC DEVELOPMENT
4.4 REIMBURSEMENT FRAMEWORK
4.5 OPPORTUNITY MAP ANALYSIS
4.6 VALUE CHAIN ANALYSIS
4.7 MICRO AND MACRO ECONOMIC FACTORS
4.7.1 CURRENT MARKET PENETRATION
4.7.2 GROWTH PROSPECTS
4.7.3 KEY PRICING STRATEGIES
4.8 TECHNOLOGY ROADMAP: MIDDLE EAST AND AFRICA POINT OF CARE TESTING
5 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: REGULATIONS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING USE OF POC TESTING IN HEALTHCARE FACILITIES
6.1.2 RISING INCIDENCE OF SUBSTANCE ABUSE
6.1.3 INCREASED ADOPTION OF TELEMEDICINE
6.1.4 ADVANCEMENTS TECHNOLOGIES ENHANCING POC TESTING WITH BIOSENSORS AND MOBILE INTEGRATION
6.2 RESTRAINTS
6.2.1 DATA SECURITY AND PRIVACY CONCERNS
6.2.2 LACK OF ACCURACY AND TECHNICAL CHALLENGES
6.3 OPPORTUNITIES
6.3.1 RISING AWARENESS AND ADVOCACY FOR POINT-OF-CARE TESTING
6.3.2 STRATEGIC INITIATION AND DECISION TAKEN BY THE MARKET PLAYERS
6.3.3 EXPANDING PRODUCT RANGE FOR POINT-OF-CARE TESTING
6.4 CHALLENGES
6.4.1 LIMITED AWARENESS AND ACCEPTANCE
6.4.2 IMPACT OF HIGH MAINTENANCE COSTS THREATENING POINT-OF-CARE TESTING (POCT) SUSTAINABILITY IN LOW-RESOURCE SETTINGS
7 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET, BY PRODUCT TYPE
7.1 OVERVIEW
7.2 GLUCOSE MONITORING PRODUCTS
7.2.1 SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES
7.2.1.1 Strips
7.2.1.2 Meters
7.2.1.3 Lancets and Lancing Devices
7.2.2 CONTINUOUS GLUCOSE MONITORING (CGM) SYSTEMS
7.3 INFECTIOUS DISEASE TESTING PRODUCTS
7.3.1 COVID-19
7.3.2 HIV TESTING PRODUCTS
7.3.2.1 Testing Reagents
7.3.2.2 Testing Equipment
7.3.3 RESPIRATORY INFECTION TESTING PRODUCTS
7.3.4 SEXUALLY TRANSMITTED DISEASES (STD) TESTING
7.3.4.1 NAAT-Based Systems
7.3.4.2 NON–NAAT-Based Systems
7.3.5 HEPATITIS C TESTING PRODUCTS
7.3.5.1 HCV Antibody Tests
7.3.5.2 HCV Viral Load Tests
7.3.6 INFLUENZA TESTING PRODUCTS
7.3.6.1 Traditional Diagnostic Test
7.3.6.2 Molecular Diagnostic Assay
7.3.6.2.1 Rapid Influenza Diagnostic Test (RIDT)
7.3.6.2.2 Direct Fluorescent Antibody Test (DFAT)
7.3.6.2.3 Viral Culture
7.3.6.2.4 Serological Assay
7.3.6.3 RT-PCR
7.3.6.4 Loop-Mediated Isothermal Amplification-Based Assay (LAMP)
7.3.6.5 Nucleic Acid Sequence-Based Amplification Test (NASBAT)
7.3.6.6 Simple Amplification-Based Assay (SAMBA)
7.3.6.7 Healthcare Associated Infection (HAI) Testing
7.3.6.8 Tropical Disease Testing Products
7.3.6.9 Other Infectious Disease Testing Products
7.4 CARDIOMETABOLIC MONITORING PRODUCTS
7.4.1 CARDIAC MARKER TESTING PRODUCTS
7.4.1.1 HSTNL
7.4.1.2 BNP
7.4.1.3 D-DIMER
7.4.1.4 CK-MB
7.4.1.5 Myoglobin
7.4.2 BLOOD GAS/ELECTROLYTE TESTING PRODUCTS
7.4.2.1 Blood Gas/Electrolyte Testing Consumables
7.4.2.2 Blood Gas/Electrolyte Testing Instruments
7.4.3 CARTRIDGES
7.4.4 REAGENTS
7.4.4.1 Portable
7.4.4.2 Benchtop
7.4.4.3 Combined Analyzers
7.4.4.4 Blood Gas Analyzers
7.4.4.5 Electrolyte Analyzers
7.4.4.6 Combined Analyzers
7.4.4.7 Blood Gas Analyzers
7.4.4.8 Electrolyte Analyzers
7.4.5 HBA1C TESTING PRODUCTS
7.4.5.1 HBA1C Testing Instruments
7.4.5.2 HBA1C Testing Consumables
7.4.5.3 POC Analyzer
7.4.5.4 ECG Device
7.4.5.5 Resting ECG Devices
7.4.5.6 Stress ECG Devices
7.4.5.7 Holter Monitors
7.5 PREGNANCY AND FERTILITY TESTING PRODUCTS
7.5.1 PREGNANCY TESTING PRODUCTS
7.5.1.1 Strips/ Dip Sticks and Cards
7.5.1.2 Mid Stream Devices
7.5.1.3 Cassettes
7.5.1.4 Digital Devices
7.5.1.5 Line-Indicator Devices
7.5.2 FERTILITY TESTING PRODUCTS
7.5.2.1 Luteinizing Hormone (LH) Urine Test
7.5.2.2 FSH Test
7.5.2.3 others
7.6 HAEMATOLOGY TESTING PRODUCTS
7.7 COAGULATION MONITORING PRODUCTS
7.7.1 ANTICOAGULATION MONITORING DEVICES
7.7.1.1 Prothrombin Time/International Normalized Ratio (PT-INR) Testing Devices
7.7.1.2 Activated Clotting Time (ACT)
7.7.1.3 Activated Partial Thromboplastin Time (APPT)
7.7.1.4 Platelet Function Monitoring Devices
7.7.1.5 Viscoelastic Coagulation Monitoring Devices
7.7.1.6 Rotational Thromboelastometry (ROTEM)
7.7.1.7 Thromboelastography (TEG)
7.7.1.8 Drug-Of-Abuse (DOA) Testing Products
7.7.2 DOA ANALYSERS
7.7.2.1 Immunoassays
7.7.2.2 Chromatographic Devices
7.7.2.3 Breath Analysers
7.7.3 RAPID TESTING DEVICES
7.7.3.1 Urine Testing Devices
7.7.3.2 Oral Fluid Testing Devices
7.7.3.4 Others
7.8 URINALYSIS TESTING PRODUCTS
7.8.1.1 POC Urine Strip Self-Testing
7.8.1.2 POC Urine Test Strip Professional Testing
7.9 CHOLESTEROL TESTING PRODUCTS
7.9.1.1 Testing Kits
7.9.1.2 Instruments
7.9.1.3 Table-Top Analyzers
7.9.1.4 Hand-Held Analyzers
7.1 TUMOR/CANCER MARKER TESTING PRODUCTS
7.11 FECAL OCCULT TESTING PRODUCTS
7.11.1.1 Guaiac FOB Stool Test
7.11.1.2 Lateral Flow Immuno-FOB Test
7.11.1.3 Immuno-FOB Agglutination Test
7.11.1.4 Immuno-FOB ELISA Test
7.12 OTHERS
8 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET, BY PLATFORM
8.1 OVERVIEW
8.2 LATERAL FLOW ASSAYS/IMMUNOCHROMATOGRAPHY TESTS
8.3 IMMUNOASSAYS
8.4 DIPSTICKS
8.5 MOLECULAR DIAGNOSTICS
8.6 CLINICAL CHEMISTRY ASSAYS
8.7 MICROFLUIDICS
8.8 HEMATOLOGY
8.9 OTHERS
9 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION
9.1 OVERVIEW
9.2 BLOOD GLUCOSE
9.3 INFECTIOUS DISEASES
9.3.1 COVID-19 TESTING
9.3.2 HIV TESTING
9.3.3 HEPATITIS C TESTING
9.3.4 INFLUENZA TESTING
9.3.5 TUBERCULOSIS TESTING
9.3.6 OTHERS
9.4 VITAL SIGN MONITORING
9.5 CARDIAC MONITORING
9.6 COAGULATION
9.7 HAEMATOLOGY
9.8 NON- INVASIVE SPO2 MONITORING
9.9 BLOOD TRANSFUSION
9.1 NON- INVASIVE PCO2 MONITORING
9.11 WHOLE BLOOD ANALYSIS
9.12 OTHERS
10 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET, BY PRESCRIPTION MODE
10.1 OVERVIEW
10.2 OTC TESTING
10.3 PRESCRIPTION-BASED TESTING
11 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL
11.1 OVERVIEW
11.2 DIRECT TENDER
11.3 RETAIL SALES
11.4 ONLINE SALES
11.5 OTHERS
12 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS
12.2.1 PRIVATE
12.2.1.1 Tier 1
12.2.1.2 Tier 2
12.2.1.3 Tier 3
12.2.2 PUBLIC
12.2.2.1 Tier 1
12.2.2.2 Tier 2
12.2.2.3 Tier 3
12.3 HOME CARE
12.4 CLINICS
12.5 LABORATORIES
12.6 DIAGNOSTIC CENTERS
12.7 PATHOLOGY LABS
12.8 AMBULATORY SURGERY CENTERS
12.9 ELDERLY CARE CENTERS
12.1 OTHERS
13 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION
13.1 MIDDLE EAST AND AFRICA
13.1.1 SAUDI ARABIA
13.1.2 SOUTH AFRICA
13.1.3 U.A.E.
13.1.4 ISRAEL
13.1.5 KUWAIT
13.1.6 EGYPT
13.1.7 REST OF MIDDLE EAST AND AFRICA
14 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: GLOBAL
15 SWOT ANALYSIS
16 COMPANY PROFILES
16.1 ABBOTT POINT OF CARE INC(ABBOTT)
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENT
16.2 SINOCARE.
16.2.1 COMPANY SNAPSHOT
16.2.2 COMPANY SHARE ANALYSIS
16.2.3 PRODUCT PORTFOLIO
16.2.4 RECENT DEVELOPMENT
16.3 F. HOFFMANN-LA ROCHE LTD
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT DEVELOPMENT
16.4 DANAHER
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT DEVELOPMENT
16.5 HOLOGIC, INC
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENT
16.6 ACCUBIOTECH CO., LTD.
16.6.1 COMPANY SNAPSHOT
16.6.2 PRODUCT PORTFOLIO
16.6.3 RECENT DEVELOPMENT
16.7 ABAXIS (ABAXIS IS A PART OF ZOETIS)
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENT
16.8 AUTOBIO
16.8.1 COMPANY SNAPSHOT
16.8.2 REVENUE ANALYSIS
16.8.3 PRODUCT PORTFOLIO
16.8.4 RECENT DEVELOPMENT
16.9 BD VERITOR(BD)
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT DEVELOPMENT
16.1 BINX HEALTH
16.10.1 COMPANY SNAPSHOT
16.10.2 SOLUTION PORTFOLIO
16.10.3 RECENT DEVELOPMENT
16.11 BIOMERIEUX
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUE ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT DEVELOPMENT
16.12 BIO- RAD LABORATORIES, INC.
16.12.1 COMPANY SNAPSHOT
16.12.2 REVENUE ANALYSIS
16.12.3 PRODUCT PORTFOLIO
16.12.4 RECENT DEVELOPMENT
16.13 CHEMBIO DIAGNOSTICS, INC.
16.13.1 COMPANY SNAPSHOT
16.13.2 PRODUCT PORTFOLIO
16.13.3 RECENT DEVELOPMENT
16.14 EKF DIAGNOSTICS HOLDINGS PLC
16.14.1 COMPANY SNAPSHOT
16.14.2 REVENUE ANALYSIS
16.14.3 PRODUCT PORTFOLIO
16.14.4 RECENT DEVELOPMENT
16.15 GETEIN BIOTECH, INC.
16.15.1 COMPANY SNAPSHOT
16.15.2 REVENUE ANALYSIS
16.15.3 PRODUCT PORTFOLIO
16.15.4 RECENT DEVELOPMENT
16.16 LAMDAGEN CORPORATION
16.16.1 COMPANY SNAPSHOT
16.16.2 PRODUCT PORTFOLIO
16.16.3 RECENT DEVELOPMENT
16.17 MERIDIAN BIOSCIENCE
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENT
16.18 NOVA BIOMEDICAL
16.18.1 COMPANY SNAPSHOT
16.18.2 PRODUCT PORTFOLIO
16.18.3 RECENT DEVELOPMENT
16.19 PFIZER INC.
16.19.1 COMPANY SNAPSHOT
16.19.2 PRODUCT PORTFOLIO
16.19.3 RECENT DEVELOPMENT
16.2 PTS DIAGNOSTICS
16.20.1 COMPANY SNAPSHOT
16.20.2 PRODUCT PORTFOLIO
16.20.3 RECENT DEVELOPMENT
16.21 QIAGEN
16.21.1 COMPANY SNAPSHOT
16.21.2 REVENUE ANALYSIS
16.21.3 PRODUCT PORTFOLIO
16.21.4 RECENT DEVELOPMENT
16.22 QUIDELORTHO CORPORATION
16.22.1 COMPANY SNAPSHOT
16.22.2 REVENUE ANALYSIS
16.22.3 PRODUCT PORTFOLIO
16.22.4 RECENT DEVELOPMENT
16.23 QUANTUMDX GROUP LTD.
16.23.1 COMPANY SNAPSHOT
16.23.2 PRODUCT PORTFOLIO
16.23.3 RECENT DEVELOPMENT
16.24 SEKISUI DIAGNOSTICS
16.24.1 COMPANY SNAPSHOT
16.24.2 PRODUCT PORTFOLIO
16.24.3 RECENT. DEVELOPMENT
16.25 SHENZHEN NEW INDUSTRY BIOMEDICAL ENGINEERING CO., LTD.
16.25.1 COMPANY SNAPSHOT
16.25.2 REVENUE ANALYSIS
16.25.3 PRODUCT PORTFOLIO
16.25.4 RECENT DEVELOPMENT
16.26 SIEMENS HEALTHINEERS AG
16.26.1 COMPANY SNAPSHOT
16.26.2 REVENUE ANALYSIS
16.26.3 PRODUCT PORTFOLIO
16.26.4 RECENT DEVELOPMENT
16.27 SIENCO, INC.
16.27.1 COMPANY SNAPSHOT
16.27.2 PRODUCT PORTFOLIO
16.27.3 RECENT UPDATES
16.28 SYSMEX CORPORATION
16.28.1 COMPANY SNAPSHOT
16.28.2 REVENUE ANALYSIS
16.28.3 PRODUCT PORTFOLIO
16.28.4 RECENT DEVELOPMENT
16.29 TRINITY BIOTECH
16.29.1 COMPANY SNAPSHOT
16.29.2 REVENUE ANALYSIS
16.29.3 PR.ODUCT PORTFOLIO
16.29.4 RECENT DEVELOPMENT
16.3 TRIVIDIA HEALTH, INC.
16.30.1 COMPANY SNAPSHOT
16.30.2 PRODUCT PORTFOLIO
16.30.3 RECENT UPDATES
16.31 THERMO FISHER SCIENTIFIC INC.
16.31.1 COMPANY SNAPSHOT
16.31.2 REVENUE ANALYSIS
16.31.3 PRODUCT PORTFOLIO
16.31.4 RECENT DEVELOPMENT
16.32 WERFEN
16.32.1 COMPANY SNAPSHOT
16.32.2 PRODUCT PORTFOLIO
16.32.3 RECENT DEVELOPMENT
16.33 WONDFO
16.33.1 COMPANY SNAPSHOT
16.33.2 REVENUE ANALYSIS
16.33.3 PRODUCT PORTFOLIO
16.33.4 RECENT DEVELOPMENT
16.34 XIAMEN BOSON BIOTECH CO., LTD.
16.34.1 COMPANY SNAPSHOT
16.34.2 PRODUCT PORTFOLIO
16.34.3 RECENT DEVELOPMENT
17 QUESTIONNAIRE
18 RELATED REPORTS
Tabellenverzeichnis
TABLE 1 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 2 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 3 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 4 MIDDLE EAST AND AFRICA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 5 MIDDLE EAST AND AFRICA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 6 MIDDLE EAST AND AFRICA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 7 MIDDLE EAST AND AFRICA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 8 MIDDLE EAST AND AFRICA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 9 MIDDLE EAST AND AFRICA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 10 MIDDLE EAST AND AFRICA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 11 MIDDLE EAST AND AFRICA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 12 MIDDLE EAST AND AFRICA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSANDS)
TABLE 13 MIDDLE EAST AND AFRICA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSANDS UNITS)
TABLE 14 MIDDLE EAST AND AFRICA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 15 MIDDLE EAST AND AFRICA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 16 MIDDLE EAST AND AFRICA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 17 MIDDLE EAST AND AFRICA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 18 MIDDLE EAST AND AFRICA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 19 MIDDLE EAST AND AFRICA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 20 MIDDLE EAST AND AFRICA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 21 MIDDLE EAST AND AFRICA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSANDS)
TABLE 22 MIDDLE EAST AND AFRICA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 23 MIDDLE EAST AND AFRICA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 24 MIDDLE EAST AND AFRICA INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 25 MIDDLE EAST AND AFRICA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 26 MIDDLE EAST AND AFRICA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 27 MIDDLE EAST AND AFRICA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 28 MIDDLE EAST AND AFRICA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 29 MIDDLE EAST AND AFRICA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 30 MIDDLE EAST AND AFRICA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 31 MIDDLE EAST AND AFRICA CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 32 MIDDLE EAST AND AFRICA CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 33 MIDDLE EAST AND AFRICA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 34 MIDDLE EAST AND AFRICA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 35 MIDDLE EAST AND AFRICA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 36 MIDDLE EAST AND AFRICA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 37 MIDDLE EAST AND AFRICA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 38 MIDDLE EAST AND AFRICA BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 39 MIDDLE EAST AND AFRICA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 40 MIDDLE EAST AND AFRICA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 41 MIDDLE EAST AND AFRICA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 42 MIDDLE EAST AND AFRICA BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 43 MIDDLE EAST AND AFRICA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 44 MIDDLE EAST AND AFRICA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 45 MIDDLE EAST AND AFRICA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 46 MIDDLE EAST AND AFRICA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSANDS)
TABLE 47 MIDDLE EAST AND AFRICA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 48 MIDDLE EAST AND AFRICA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 49 MIDDLE EAST AND AFRICA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 50 MIDDLE EAST AND AFRICA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 51 MIDDLE EAST AND AFRICA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 52 MIDDLE EAST AND AFRICA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 53 MIDDLE EAST AND AFRICA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 54 MIDDLE EAST AND AFRICA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 55 MIDDLE EAST AND AFRICA PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 56 MIDDLE EAST AND AFRICA PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 57 MIDDLE EAST AND AFRICA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 58 MIDDLE EAST AND AFRICA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSANDS UNITS)
TABLE 59 MIDDLE EAST AND AFRICA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 60 MIDDLE EAST AND AFRICA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 61 MIDDLE EAST AND AFRICA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 62 MIDDLE EAST AND AFRICA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 63 MIDDLE EAST AND AFRICA HAEMATOLOGY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 64 MIDDLE EAST AND AFRICA COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSANDS)
TABLE 65 MIDDLE EAST AND AFRICA COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSANDS)
TABLE 66 MIDDLE EAST AND AFRICA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 67 MIDDLE EAST AND AFRICA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 68 MIDDLE EAST AND AFRICA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 69 MIDDLE EAST AND AFRICA VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2033 (USD THOUSANDS)
TABLE 70 MIDDLE EAST AND AFRICA DRUGS-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 71 MIDDLE EAST AND AFRICA DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 72 MIDDLE EAST AND AFRICA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSANDS)
TABLE 73 MIDDLE EAST AND AFRICA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 74 MIDDLE EAST AND AFRICA DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 75 MIDDLE EAST AND AFRICA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 76 MIDDLE EAST AND AFRICA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 77 MIDDLE EAST AND AFRICA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 78 MIDDLE EAST AND AFRICA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 79 MIDDLE EAST AND AFRICA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 80 MIDDLE EAST AND AFRICA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 81 MIDDLE EAST AND AFRICA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 82 MIDDLE EAST AND AFRICA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 83 MIDDLE EAST AND AFRICA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 84 MIDDLE EAST AND AFRICA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSANDS UNITS)
TABLE 85 MIDDLE EAST AND AFRICA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 86 MIDDLE EAST AND AFRICA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSANDS)
TABLE 87 MIDDLE EAST AND AFRICA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 88 MIDDLE EAST AND AFRICA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 89 MIDDLE EAST AND AFRICA TUMOR/CANCER MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 90 MIDDLE EAST AND AFRICA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 91 MIDDLE EAST AND AFRICA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 92 MIDDLE EAST AND AFRICA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSANDS UNITS)
TABLE 93 MIDDLE EAST AND AFRICA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 94 MIDDLE EAST AND AFRICA OTHERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 95 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2033 (USD THOUSAND)
TABLE 96 MIDDLE EAST AND AFRICA LATERAL FLOW ASSAYS/IMMUNOCHROMATOGRAPHY TESTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSANDS)
TABLE 97 MIDDLE EAST AND AFRICA IMMUNOASSAYS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSANDS)
TABLE 98 MIDDLE EAST AND AFRICA DIPSTICKS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSANDS)
TABLE 99 MIDDLE EAST AND AFRICA MOLECULAR DIAGNOSTICS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSANDS)
TABLE 100 MIDDLE EAST AND AFRICA CLINICAL CHEMISTRY ASSAYS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 101 MIDDLE EAST AND AFRICA MICROFLUIDICS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSANDS)
TABLE 102 MIDDLE EAST AND AFRICA HEMATOLOGY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSANDS)
TABLE 103 MIDDLE EAST AND AFRICA OTHERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSANDS)
TABLE 104 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2033 (USD THOUSAND)
TABLE 105 MIDDLE EAST AND AFRICA BLOOD GLUCOSE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 106 MIDDLE EAST AND AFRICA INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 107 MIDDLE EAST AND AFRICA INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 108 MIDDLE EAST AND AFRICA VITAL SIGN MONITORING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 109 MIDDLE EAST AND AFRICA CARDIAC MONITORING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 110 MIDDLE EAST AND AFRICA COAGULATION IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 111 MIDDLE EAST AND AFRICA HAEMATOLOGY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 112 MIDDLE EAST AND AFRICA NON- INVASIVE SPO2 MONITORING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 113 MIDDLE EAST AND AFRICA BLOOD TRANSFUSION IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 114 MIDDLE EAST AND AFRICA NON- INVASIVE PCO2 MONITORING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 115 MIDDLE EAST AND AFRICA WHOLE BLOOD ANALYSIS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 116 MIDDLE EAST AND AFRICA OTHERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 117 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2033 (USD THOUSANDS)
TABLE 118 MIDDLE EAST AND AFRICA OTC TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 119 MIDDLE EAST AND AFRICA PRESCRIPTION-BASED TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSANDS)
TABLE 120 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2033 (USD MILLION)
TABLE 121 MIDDLE EAST AND AFRICA DIRECT TENDER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD MILLIONS)
TABLE 122 MIDDLE EAST AND AFRICA RETAIL SALES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD MILLIONS)
TABLE 123 MIDDLE EAST AND AFRICA ONLINE SALES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD MILLIONS)
TABLE 124 MIDDLE EAST AND AFRICA OTHERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD MILLIONS)
TABLE 125 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 126 MIDDLE EAST AND AFRICA HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2032 (USD THOUSANDS)
TABLE 127 MIDDLE EAST AND AFRICA HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSANDS)
TABLE 128 MIDDLE EAST AND AFRICA PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSANDS)
TABLE 129 MIDDLE EAST AND AFRICA PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSANDS)
TABLE 130 MIDDLE EAST AND AFRICA HOME CARE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 131 MIDDLE EAST AND AFRICA CLINICS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 132 MIDDLE EAST AND AFRICA LABORATORIES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 133 MIDDLE EAST AND AFRICA DIAGNOSTIC CENTERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 134 MIDDLE EAST AND AFRICA PATHOLOGY LABS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 135 MIDDLE EAST AND AFRICA AMBULATORY SURGERY CENTERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 136 MIDDLE EAST AND AFRICA ELDERLY CARE CENTERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 137 MIDDLE EAST AND AFRICA OTHERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 138 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY COUNTRY, 2018-2033 (USD THOUSAND)
TABLE 139 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY COUNTRY, 2018-2033 (USD THOUSAND)
TABLE 140 MIDDLE EAST AND AFRICA
TABLE 141 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 142 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 143 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 144 MIDDLE EAST AND AFRICA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 145 MIDDLE EAST AND AFRICA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 146 MIDDLE EAST AND AFRICA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 147 MIDDLE EAST AND AFRICA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 148 MIDDLE EAST AND AFRICA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 149 MIDDLE EAST AND AFRICA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 150 MIDDLE EAST AND AFRICA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 151 MIDDLE EAST AND AFRICA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 152 MIDDLE EAST AND AFRICA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 153 MIDDLE EAST AND AFRICA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 154 MIDDLE EAST AND AFRICA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 155 MIDDLE EAST AND AFRICA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 156 MIDDLE EAST AND AFRICA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 157 MIDDLE EAST AND AFRICA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 158 MIDDLE EAST AND AFRICA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 159 MIDDLE EAST AND AFRICA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 160 MIDDLE EAST AND AFRICA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 161 MIDDLE EAST AND AFRICA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 162 MIDDLE EAST AND AFRICA INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 163 MIDDLE EAST AND AFRICA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 164 MIDDLE EAST AND AFRICA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 165 MIDDLE EAST AND AFRICA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 166 MIDDLE EAST AND AFRICA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 167 MIDDLE EAST AND AFRICA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 168 MIDDLE EAST AND AFRICA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 169 MIDDLE EAST AND AFRICA CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 170 MIDDLE EAST AND AFRICA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 171 MIDDLE EAST AND AFRICA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 172 MIDDLE EAST AND AFRICA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 173 MIDDLE EAST AND AFRICA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 174 MIDDLE EAST AND AFRICA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 175 MIDDLE EAST AND AFRICA BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 176 MIDDLE EAST AND AFRICA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 177 MIDDLE EAST AND AFRICA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 178 MIDDLE EAST AND AFRICA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 179 MIDDLE EAST AND AFRICA BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 180 MIDDLE EAST AND AFRICA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 181 MIDDLE EAST AND AFRICA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 182 MIDDLE EAST AND AFRICA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 183 MIDDLE EAST AND AFRICA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 184 MIDDLE EAST AND AFRICA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 185 MIDDLE EAST AND AFRICA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 186 MIDDLE EAST AND AFRICA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 187 MIDDLE EAST AND AFRICA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 188 MIDDLE EAST AND AFRICA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 189 MIDDLE EAST AND AFRICA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 190 MIDDLE EAST AND AFRICA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 191 MIDDLE EAST AND AFRICA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 192 MIDDLE EAST AND AFRICA PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 193 MIDDLE EAST AND AFRICA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 194 MIDDLE EAST AND AFRICA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 195 MIDDLE EAST AND AFRICA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 196 MIDDLE EAST AND AFRICA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 197 MIDDLE EAST AND AFRICA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 198 MIDDLE EAST AND AFRICA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 199 MIDDLE EAST AND AFRICA COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 200 MIDDLE EAST AND AFRICA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 201 MIDDLE EAST AND AFRICA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 202 MIDDLE EAST AND AFRICA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 203 MIDDLE EAST AND AFRICA VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2033 (USD THOUSAND)
TABLE 204 MIDDLE EAST AND AFRICA DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 205 MIDDLE EAST AND AFRICA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 206 MIDDLE EAST AND AFRICA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 207 MIDDLE EAST AND AFRICA DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 208 MIDDLE EAST AND AFRICA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 209 MIDDLE EAST AND AFRICA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 210 MIDDLE EAST AND AFRICA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 211 MIDDLE EAST AND AFRICA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 212 MIDDLE EAST AND AFRICA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 213 MIDDLE EAST AND AFRICA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 214 MIDDLE EAST AND AFRICA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 215 MIDDLE EAST AND AFRICA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 216 MIDDLE EAST AND AFRICA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 217 MIDDLE EAST AND AFRICA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 218 MIDDLE EAST AND AFRICA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 219 MIDDLE EAST AND AFRICA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 220 MIDDLE EAST AND AFRICA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 221 MIDDLE EAST AND AFRICA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 222 MIDDLE EAST AND AFRICA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 223 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2033 (USD THOUSAND)
TABLE 224 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2033 (USD THOUSAND)
TABLE 225 MIDDLE EAST AND AFRICA INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 226 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2033 (USD THOUSAND)
TABLE 227 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 228 MIDDLE EAST AND AFRICA HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 229 MIDDLE EAST AND AFRICA PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 230 MIDDLE EAST AND AFRICA PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 231 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2033 (USD THOUSAND)
TABLE 232 SAUDI ARABIA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 233 SAUDI ARABIA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 234 SAUDI ARABIA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 235 SAUDI ARABIA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 236 SAUDI ARABIA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 237 SAUDI ARABIA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 238 SAUDI ARABIA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 239 SAUDI ARABIA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 240 SAUDI ARABIA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 241 SAUDI ARABIA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 242 SAUDI ARABIA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 243 SAUDI ARABIA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 244 SAUDI ARABIA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 245 SAUDI ARABIA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 246 SAUDI ARABIA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 247 SAUDI ARABIA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 248 SAUDI ARABIA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 249 SAUDI ARABIA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 250 SAUDI ARABIA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 251 SAUDI ARABIA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 252 SAUDI ARABIA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 253 SAUDI ARABIA INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 254 SAUDI ARABIA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 255 SAUDI ARABIA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 256 SAUDI ARABIA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 257 SAUDI ARABIA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 258 SAUDI ARABIA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 259 SAUDI ARABIA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 260 SAUDI ARABIA CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 261 SAUDI ARABIA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 262 SAUDI ARABIA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 263 SAUDI ARABIA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 264 SAUDI ARABIA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 265 SAUDI ARABIA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 266 SAUDI ARABIA BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 267 SAUDI ARABIA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 268 SAUDI ARABIA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 269 SAUDI ARABIA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 270 SAUDI ARABIA BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 271 SAUDI ARABIA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 272 SAUDI ARABIA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 273 SAUDI ARABIA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 274 SAUDI ARABIA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 275 SAUDI ARABIA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 276 SAUDI ARABIA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 277 SAUDI ARABIA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 278 SAUDI ARABIA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 279 SAUDI ARABIA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 280 SAUDI ARABIA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 281 SAUDI ARABIA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 282 SAUDI ARABIA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 283 SAUDI ARABIA PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 284 SAUDI ARABIA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 285 SAUDI ARABIA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 286 SAUDI ARABIA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 287 SAUDI ARABIA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 288 SAUDI ARABIA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 289 SAUDI ARABIA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 290 SAUDI ARABIA COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 291 SAUDI ARABIA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 292 SAUDI ARABIA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 293 SAUDI ARABIA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 294 SAUDI ARABIA VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2033 (USD THOUSAND)
TABLE 295 SAUDI ARABIA DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 296 SAUDI ARABIA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 297 SAUDI ARABIA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 298 SAUDI ARABIA DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 299 SAUDI ARABIA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 300 SAUDI ARABIA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 301 SAUDI ARABIA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 302 SAUDI ARABIA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 303 SAUDI ARABIA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 304 SAUDI ARABIA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 305 SAUDI ARABIA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 306 SAUDI ARABIA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 307 SAUDI ARABIA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 308 SAUDI ARABIA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 309 SAUDI ARABIA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 310 SAUDI ARABIA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 311 SAUDI ARABIA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 312 SAUDI ARABIA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 313 SAUDI ARABIA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 314 SAUDI ARABIA POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2033 (USD THOUSAND)
TABLE 315 SAUDI ARABIA POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2033 (USD THOUSAND)
TABLE 316 SAUDI ARABIA INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 317 SAUDI ARABIA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2033 (USD THOUSAND)
TABLE 318 SAUDI ARABIA POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 319 SAUDI ARABIA HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 320 SAUDI ARABIA PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 321 SAUDI ARABIA PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 322 SAUDI ARABIA POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2033 (USD THOUSAND)
TABLE 323 SOUTH AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 324 SOUTH AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 325 SOUTH AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 326 SOUTH AFRICA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 327 SOUTH AFRICA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 328 SOUTH AFRICA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 329 SOUTH AFRICA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 330 SOUTH AFRICA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 331 SOUTH AFRICA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 332 SOUTH AFRICA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 333 SOUTH AFRICA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 334 SOUTH AFRICA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 335 SOUTH AFRICA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 336 SOUTH AFRICA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 337 SOUTH AFRICA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 338 SOUTH AFRICA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 339 SOUTH AFRICA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 340 SOUTH AFRICA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 341 SOUTH AFRICA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 342 SOUTH AFRICA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 343 SOUTH AFRICA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 344 SOUTH AFRICA INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 345 SOUTH AFRICA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 346 SOUTH AFRICA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 347 SOUTH AFRICA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 348 SOUTH AFRICA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 349 SOUTH AFRICA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 350 SOUTH AFRICA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 351 SOUTH AFRICA CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 352 SOUTH AFRICA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 353 SOUTH AFRICA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 354 SOUTH AFRICA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 355 SOUTH AFRICA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 356 SOUTH AFRICA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 357 SOUTH AFRICA BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 358 SOUTH AFRICA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 359 SOUTH AFRICA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 360 SOUTH AFRICA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 361 SOUTH AFRICA BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 362 SOUTH AFRICA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 363 SOUTH AFRICA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 364 SOUTH AFRICA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 365 SOUTH AFRICA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 366 SOUTH AFRICA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 367 SOUTH AFRICA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 368 SOUTH AFRICA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 369 SOUTH AFRICA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 370 SOUTH AFRICA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 371 SOUTH AFRICA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 372 SOUTH AFRICA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 373 SOUTH AFRICA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 374 SOUTH AFRICA PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 375 SOUTH AFRICA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 376 SOUTH AFRICA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 377 SOUTH AFRICA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 378 SOUTH AFRICA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 379 SOUTH AFRICA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 380 SOUTH AFRICA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 381 SOUTH AFRICA COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 382 SOUTH AFRICA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 383 SOUTH AFRICA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 384 SOUTH AFRICA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 385 SOUTH AFRICA VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2033 (USD THOUSAND)
TABLE 386 SOUTH AFRICA DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 387 SOUTH AFRICA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 388 SOUTH AFRICA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 389 SOUTH AFRICA DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 390 SOUTH AFRICA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 391 SOUTH AFRICA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 392 SOUTH AFRICA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 393 SOUTH AFRICA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 394 SOUTH AFRICA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 395 SOUTH AFRICA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 396 SOUTH AFRICA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 397 SOUTH AFRICA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 398 SOUTH AFRICA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 399 SOUTH AFRICA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 400 SOUTH AFRICA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 401 SOUTH AFRICA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 402 SOUTH AFRICA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 403 SOUTH AFRICA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 404 SOUTH AFRICA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 405 SOUTH AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2033 (USD THOUSAND)
TABLE 406 SOUTH AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2033 (USD THOUSAND)
TABLE 407 SOUTH AFRICA INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 408 SOUTH AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2033 (USD THOUSAND)
TABLE 409 SOUTH AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 410 SOUTH AFRICA HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 411 SOUTH AFRICA PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 412 SOUTH AFRICA PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 413 SOUTH AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2033 (USD THOUSAND)
TABLE 414 U.A.E POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 415 U.A.E POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 416 U.A.E POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 417 U.A.E GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 418 U.A.E GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 419 U.A.E GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 420 U.A.E SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 421 U.A.E SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 422 U.A.E SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 423 U.A.E INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 424 U.A.E INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 425 U.A.E INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 426 U.A.E HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 427 U.A.E HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 428 U.A.E HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 429 U.A.E SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 430 U.A.E SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 431 U.A.E SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 432 U.A.E HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 433 U.A.E HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 434 U.A.E HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 435 U.A.E INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 436 U.A.E TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 437 U.A.E TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 438 U.A.E TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 439 U.A.E MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 440 U.A.E MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 441 U.A.E MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 442 U.A.E CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 443 U.A.E POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 444 U.A.E POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 445 U.A.E CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 446 U.A.E CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 447 U.A.E CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 448 U.A.E BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 449 U.A.E BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 450 U.A.E BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 451 U.A.E BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 452 U.A.E BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 453 U.A.E PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 454 U.A.E PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 455 U.A.E PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 456 U.A.E BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 457 U.A.E BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 458 U.A.E BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 459 U.A.E HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 460 U.A.E HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 461 U.A.E HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 462 U.A.E ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 463 U.A.E ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 464 U.A.E ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 465 U.A.E PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 466 U.A.E PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 467 U.A.E PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 468 U.A.E PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 469 U.A.E FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 470 U.A.E FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 471 U.A.E FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 472 U.A.E COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 473 U.A.E ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 474 U.A.E ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 475 U.A.E ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 476 U.A.E VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2033 (USD THOUSAND)
TABLE 477 U.A.E DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 478 U.A.E DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 479 U.A.E DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 480 U.A.E DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 481 U.A.E RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 482 U.A.E RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 483 U.A.E RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 484 U.A.E URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 485 U.A.E URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 486 U.A.E URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 487 U.A.E CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 488 U.A.E CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 489 U.A.E CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 490 U.A.E INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 491 U.A.E INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 492 U.A.E INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 493 U.A.E FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 494 U.A.E FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 495 U.A.E FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 496 U.A.E POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2033 (USD THOUSAND)
TABLE 497 U.A.E POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2033 (USD THOUSAND)
TABLE 498 U.A.E INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 499 U.A.E POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2033 (USD THOUSAND)
TABLE 500 U.A.E POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 501 U.A.E HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 502 U.A.E PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 503 U.A.E PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 504 U.A.E POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2033 (USD THOUSAND)
TABLE 505 ISRAEL POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 506 ISRAEL POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 507 ISRAEL POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 508 ISRAEL GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 509 ISRAEL GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 510 ISRAEL GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 511 ISRAEL SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 512 ISRAEL SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 513 ISRAEL SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 514 ISRAEL INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 515 ISRAEL INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 516 ISRAEL INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 517 ISRAEL HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 518 ISRAEL HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 519 ISRAEL HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 520 ISRAEL SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 521 ISRAEL SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 522 ISRAEL SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 523 ISRAEL HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 524 ISRAEL HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 525 ISRAEL HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 526 ISRAEL INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 527 ISRAEL TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 528 ISRAEL TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 529 ISRAEL TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 530 ISRAEL MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 531 ISRAEL MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 532 ISRAEL MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 533 ISRAEL CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 534 ISRAEL POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 535 ISRAEL POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 536 ISRAEL CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 537 ISRAEL CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 538 ISRAEL CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 539 ISRAEL BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 540 ISRAEL BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 541 ISRAEL BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 542 ISRAEL BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 543 ISRAEL BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 544 ISRAEL PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 545 ISRAEL PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 546 ISRAEL PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 547 ISRAEL BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 548 ISRAEL BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 549 ISRAEL BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 550 ISRAEL HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 551 ISRAEL HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 552 ISRAEL HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 553 ISRAEL ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 554 ISRAEL ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 555 ISRAEL ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 556 ISRAEL PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 557 ISRAEL PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 558 ISRAEL PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 559 ISRAEL PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 560 ISRAEL FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 561 ISRAEL FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 562 ISRAEL FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 563 ISRAEL COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 564 ISRAEL ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 565 ISRAEL ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 566 ISRAEL ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 567 ISRAEL VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2033 (USD THOUSAND)
TABLE 568 ISRAEL DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 569 ISRAEL DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 570 ISRAEL DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 571 ISRAEL DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 572 ISRAEL RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 573 ISRAEL RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 574 ISRAEL RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 575 ISRAEL URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 576 ISRAEL URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 577 ISRAEL URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 578 ISRAEL CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 579 ISRAEL CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 580 ISRAEL CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 581 ISRAEL INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 582 ISRAEL INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 583 ISRAEL INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 584 ISRAEL FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 585 ISRAEL FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 586 ISRAEL FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 587 ISRAEL POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2033 (USD THOUSAND)
TABLE 588 ISRAEL POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2033 (USD THOUSAND)
TABLE 589 ISRAEL INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 590 ISRAEL POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2033 (USD THOUSAND)
TABLE 591 ISRAEL POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 592 ISRAEL HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 593 ISRAEL PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 594 ISRAEL PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 595 ISRAEL POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2033 (USD THOUSAND)
TABLE 596 KUWAIT POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 597 KUWAIT POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 598 KUWAIT POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 599 KUWAIT GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 600 KUWAIT GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 601 KUWAIT GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 602 KUWAIT SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 603 KUWAIT SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 604 KUWAIT SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 605 KUWAIT INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 606 KUWAIT INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 607 KUWAIT INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 608 KUWAIT HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 609 KUWAIT HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 610 KUWAIT HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 611 KUWAIT SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 612 KUWAIT SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 613 KUWAIT SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 614 KUWAIT HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 615 KUWAIT HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 616 KUWAIT HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 617 KUWAIT INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 618 KUWAIT TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 619 KUWAIT TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 620 KUWAIT TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 621 KUWAIT MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 622 KUWAIT MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 623 KUWAIT MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 624 KUWAIT CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 625 KUWAIT POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 626 KUWAIT POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 627 KUWAIT CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 628 KUWAIT CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 629 KUWAIT CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 630 KUWAIT BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 631 KUWAIT BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 632 KUWAIT BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 633 KUWAIT BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 634 KUWAIT BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 635 KUWAIT PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 636 KUWAIT PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 637 KUWAIT PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 638 KUWAIT BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 639 KUWAIT BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 640 KUWAIT BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 641 KUWAIT HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 642 KUWAIT HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 643 KUWAIT HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 644 KUWAIT ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 645 KUWAIT ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 646 KUWAIT ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 647 KUWAIT PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 648 KUWAIT PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 649 KUWAIT PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 650 KUWAIT PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 651 KUWAIT FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 652 KUWAIT FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 653 KUWAIT FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 654 KUWAIT COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 655 KUWAIT ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 656 KUWAIT ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 657 KUWAIT ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 658 KUWAIT VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2033 (USD THOUSAND)
TABLE 659 KUWAIT DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 660 KUWAIT DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 661 KUWAIT DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 662 KUWAIT DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 663 KUWAIT RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 664 KUWAIT RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 665 KUWAIT RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 666 KUWAIT URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 667 KUWAIT URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 668 KUWAIT URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 669 KUWAIT CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 670 KUWAIT CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 671 KUWAIT CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 672 KUWAIT INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 673 KUWAIT INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 674 KUWAIT INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 675 KUWAIT FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 676 KUWAIT FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 677 KUWAIT FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 678 KUWAIT POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2033 (USD THOUSAND)
TABLE 679 KUWAIT POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2033 (USD THOUSAND)
TABLE 680 KUWAIT INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 681 KUWAIT POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2033 (USD THOUSAND)
TABLE 682 KUWAIT POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 683 KUWAIT HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 684 KUWAIT PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 685 KUWAIT PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 686 KUWAIT POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2033 (USD THOUSAND)
TABLE 687 EGYPT POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 688 EGYPT POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 689 EGYPT POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 690 EGYPT GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 691 EGYPT GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 692 EGYPT GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 693 EGYPT SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 694 EGYPT SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 695 EGYPT SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 696 EGYPT INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 697 EGYPT INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 698 EGYPT INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 699 EGYPT HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 700 EGYPT HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 701 EGYPT HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 702 EGYPT SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 703 EGYPT SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 704 EGYPT SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 705 EGYPT HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 706 EGYPT HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 707 EGYPT HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 708 EGYPT INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 709 EGYPT TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 710 EGYPT TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 711 EGYPT TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 712 EGYPT MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 713 EGYPT MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 714 EGYPT MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 715 EGYPT CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 716 EGYPT POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 717 EGYPT POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 718 EGYPT CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 719 EGYPT CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 720 EGYPT CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 721 EGYPT BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 722 EGYPT BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 723 EGYPT BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 724 EGYPT BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 725 EGYPT BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 726 EGYPT PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 727 EGYPT PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 728 EGYPT PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 729 EGYPT BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 730 EGYPT BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 731 EGYPT BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 732 EGYPT HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 733 EGYPT HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 734 EGYPT HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 735 EGYPT ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 736 EGYPT ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 737 EGYPT ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 738 EGYPT PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 739 EGYPT PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 740 EGYPT PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 741 EGYPT PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 742 EGYPT FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 743 EGYPT FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 744 EGYPT FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 745 EGYPT COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 746 EGYPT ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 747 EGYPT ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 748 EGYPT ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 749 EGYPT VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2033 (USD THOUSAND)
TABLE 750 EGYPT DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 751 EGYPT DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 752 EGYPT DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 753 EGYPT DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 754 EGYPT RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 755 EGYPT RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 756 EGYPT RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 757 EGYPT URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 758 EGYPT URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 759 EGYPT URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 760 EGYPT CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 761 EGYPT CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 762 EGYPT CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 763 EGYPT INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 764 EGYPT INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 765 EGYPT INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 766 EGYPT FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 767 EGYPT FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 768 EGYPT FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 769 EGYPT POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2033 (USD THOUSAND)
TABLE 770 EGYPT POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2033 (USD THOUSAND)
TABLE 771 EGYPT INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 772 EGYPT POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2033 (USD THOUSAND)
TABLE 773 EGYPT POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 774 EGYPT HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 775 EGYPT PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 776 EGYPT PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 777 EGYPT POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2033 (USD THOUSAND)
TABLE 778 REST OF MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 779 REST OF MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 780 REST OF MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
Abbildungsverzeichnis
FIGURE 1 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET: REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET: DBMR MARKET POSITION GRID
FIGURE 8 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET: MARKET END USER COVERAGE GRID
FIGURE 9 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET: SEGMENTATION
FIGURE 11 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET EXECUTIVE SUMMARY
FIGURE 12 STRATEGIC DECISIONS
FIGURE 13 RISING USE OF POC TESTING IN HEALTHCARE FACILITIES IS EXPECTED TO DRIVE THE GROWTH OF MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET FROM 2026 TO 2033
FIGURE 14 THE GLUCOSE MONITORING PRODUCTS SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET IN 2026 - 2032
FIGURE 15 DRCO ANALYSIS
FIGURE 16 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, 2025
FIGURE 17 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, 2026-2033 (USD THOUSAND)
FIGURE 18 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, CAGR (2026-2033)
FIGURE 19 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 20 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, 2025
FIGURE 21 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, 2026 TO 2033 (USD THOUSAND)
FIGURE 22 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, CAGR (2026- 2033)
FIGURE 23 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, LIFELINE CURVE
FIGURE 24 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, 2025
FIGURE 25 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, 2026 TO 2033 (USD THOUSAND)
FIGURE 26 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, CAGR (2026- 2033)
FIGURE 27 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 28 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, 2025
FIGURE 29 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, 2026 TO 2033 (USD THOUSAND)
FIGURE 30 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, CAGR (2026- 2033)
FIGURE 31 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, LIFELINE CURVE
FIGURE 32 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, 2025
FIGURE 33 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, 2026 TO 2033 (USD THOUSAND)
FIGURE 34 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2026- 2033)
FIGURE 35 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 36 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, 2025
FIGURE 37 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, 2026 TO 2033 (USD THOUSAND)
FIGURE 38 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, CAGR (2026- 2033)
FIGURE 39 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, LIFELINE CURVE
FIGURE 40 MIDDLE EAST AND AFRICA POINT-OF-CARE-TESTING (POCT) MARKET: SNAPSHOT
FIGURE 41 MIDDLE EAST AND AFRICA POINT-OF-CARE TESTING (POCT) MARKET: COMPANY SHARE 2025 (%)
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.