North America Anesthesia Devices Market
Marktgröße in Milliarden USD
CAGR :
% 
 3.80
6.50
2024
2032
3.80
6.50
2024
2032
| 2025 –2032 | |
| USD 3.80 | |
| USD 6.50 | |
|
|
|
|
North America Anesthesia Devices Market Segmentation, By Product (Anesthesia Delivery Machines, Anesthesia Disposables and Accessories, Anesthesia Monitors and Anesthesia Information Management Systems (Aims)), Type (General Anesthesia and Local Anesthesia), Application (Cardiology, Neurology, Dental, Ophthalmology, Urology, Orthopaedics and Others), End User (Hospitals, Ambulatory Service Centres, Clinics and Others)- Industry Trends and Forecast to 2032
Anesthesia Devices Market Size
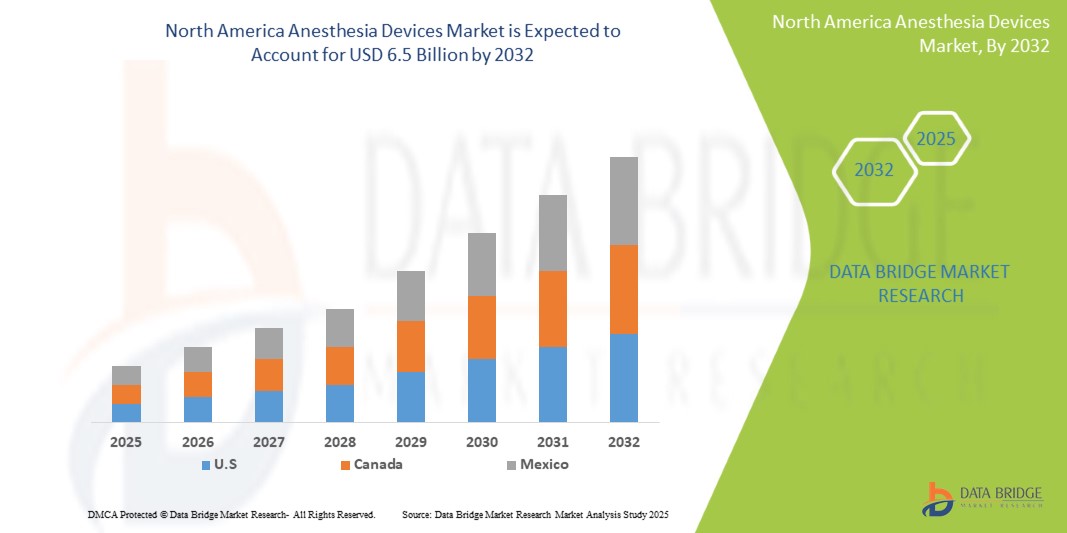
- The North America Anesthesia Devices Market was valued atUSD 3.8 Billion in 2024and is expected to reachUSD 6.5 Billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at aCAGR of 7.1%primarily driven by the anticipated launch of therapies
- The drivers of the Anesthesia Devices Market include the Growing Demand for Safe and Effective Anesthesia Solutions, technological Advancements in Anesthesia Devices and increase in Surgical Procedures and Diagnostic Tests.
North America Anesthesia Devices Market Analysis
- Anesthesia Devices play a crucial role in healthcare by enabling safe and effective anesthesia delivery during a wide range of surgical procedures. These devices are essential in improving patient safety, reducing surgical risks, and enhancing the overall quality of medical treatments across various specialties such as cardiology, orthopedics, and neurology.
- The demand for Anesthesia Devices in North America is driven by factors such as technological advancements, increasing surgical procedures, and a growing aging population. Additionally, the high levels of healthcare expenditure, along with a robust healthcare infrastructure, support the widespread adoption of advanced anesthesia technologies across hospitals and surgical centers.
- North America stands as a dominant region in the global Anesthesia Devices market, with the United States leading in market share due to its well-established healthcare system, continuous medical innovation, and significant investments in healthcare infrastructure. The presence of major industry players and increasing governmental focus on improving healthcare access further bolsters market growth.
- For instance, North America accounted for 40.8% of the global anesthesia devices market, with the U.S. contributing significantly to this share. In 2025, the market is expected to reach USD 6.04 billion and grow to USD 8.51 billion by 2030, driven by increased surgical procedures and advanced anesthesia technologies.
- Globally, Anesthesia Devices such as anesthesia delivery machines, ventilators, and anesthesia monitoring systems are pivotal in ensuring safe anesthesia administration during surgeries. In North America, the adoption of these devices is supported by continuous healthcare reforms, an aging population requiring more surgeries, and the increasing demand for minimally invasive surgical procedures, all contributing to the expansion of the anesthesia devices market
Report ScopeAnesthesia DevicesMarket Segmentation
|
Attributes |
Anesthesia DevicesKeyMarket Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Anesthesia Devices Market Trends
“Integration of AI and Advanced Monitoring Technologies”
- Adoption of AI-Enhanced Monitoring: The North American market is witnessing a surge in the integration of artificial intelligence (AI) into anesthesia monitoring devices. These advancements enable real-time data analysis, enhancing patient safety and optimizing anesthesia delivery during surgical procedures.
- Shift Towards Minimally Invasive Procedures: There's a growing preference for minimally invasive surgeries, which require sophisticated anesthesia devices capable of precise control and monitoring, thereby driving demand for advanced equipment in the region.
Anesthesia Devices Market Dynamics
Driver
“Rising Surgical Procedures and Technological Advancements”
- Increase in Surgical Interventions: The escalating number of surgical procedures, particularly among the aging population and patients with chronic diseases, is propelling the demand for anesthesia devices in North America.
- Technological Innovations: Continuous advancements in anesthesia technology, including the development of user-friendly workstations and integration with electronic health records, are enhancing operational efficiency and patient outcomes.
Opportunity
“Expansion of Ambulatory Surgical Centers and Telehealth Services”
- Growth of Outpatient Facilities: The proliferation of ambulatory surgical centers (ASCs) is creating new avenues for anesthesia device manufacturers, as these centers require compact, efficient, and cost-effective equipment.
- Telehealth Integration: The expansion of telehealth services is facilitating remote monitoring and management of anesthesia care, opening opportunities for devices that can seamlessly integrate with telemedicine platforms.
Restraint/Challenge
“High Costs and Regulatory Hurdles”
- Economic Constraints: The substantial costs associated with advanced anesthesia devices can be a barrier for smaller healthcare facilities and ASCs, potentially limiting market penetration.
- Regulatory Compliance: Navigating the complex regulatory landscape, including obtaining necessary approvals and adhering to stringent standards, poses challenges for manufacturers aiming to introduce new products in the North American market.
Anesthesia Devices Market Scope
The market is segmented on the basis, product, type, and end user.
|
Segmentation |
Sub-Segmentation |
|
By Product Type |
|
|
By Technology |
|
|
By Application |
|
|
ByDistribution Channel
|
|
Anesthesia Devices Market Regional Analysis
“U.S. is the Dominant Country in the Anesthesia Devices Market”
- North America leads the global Anesthesia Devices market, driven by its advanced healthcare infrastructure, high surgical procedure volumes, and the presence of leading medical device manufacturers such as GE Healthcare, Medtronic, and Baxter International.
- The United States holds the largest market share, fueled by the rising prevalence of chronic diseases requiring surgical interventions, strong hospital networks, and a high adoption rate of technologically advanced anesthesia systems.
- Policies such as the Affordable Care Act (ACA), which mandate broader healthcare coverage, indirectly support the market by ensuring access to elective and emergency surgeries that require anesthesia support.
- Robust investments in R&D, along with the integration of digital technologies like artificial intelligence (AI) and electronic health records (EHR) into anesthesia management systems, are further boosting the country’s leadership in the market
“Canada is Projected to Register the Highest Growth Rate”
- Canada is expected to witness the fastest growth in the North American Anesthesia Devices market, supported by its publicly funded universal healthcare system and growing number of surgical procedures across provinces.
- The country’s proactive healthcare strategies, including increased funding for hospitals and surgical centers, are driving the demand for anesthesia workstations, ventilators, and monitoring equipment.
- Urban regions such as Toronto, Vancouver, and Montreal are seeing significant installations of high-end anesthesia systems due to the rise in outpatient surgeries and specialized surgical care facilities.
- Canada’s healthcare sector is increasingly adopting remote patient monitoring and smart anesthesia technologies, spurred by government incentives and a push toward digital health transformation, further expanding market potential
Anesthesia Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Medtronic (Ireland)
- GE Healthcare (U.S)
- Drägerwerk AG & Co. KGaA (Germany)
- Philips Healthcare (Amsterdam, Netherlands)
- Smiths Medical (Minneapolis, U.S)
- Masimo Corporation (Irvine, U.S)
- Johnson & Johnson (U.S)
- Fisher & Paykel Healthcare (New Zealand)
- Baxter International Inc. (U.S)
- Aesculap Inc. (A B. Braun Company) (U.S)
Latest Developments in Global Anesthesia Devices Market
- In April 2022, GE Healthcare received the FDA pre-market approval (PMA) for its End-tidal (Et) Control software for general anesthesia delivery on its Aisys CS2Anesthesia Delivery System.
- In May 2022, Fisher & Paykel Healthcare expands offering in anesthesia with the release of the Optiflow Switch and Optiflow Trace
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.