North America Biopsy Devices Market
Marktgröße in Milliarden USD
CAGR :
% 
 USD
1.80 Billion
USD
3.60 Billion
2024
2032
USD
1.80 Billion
USD
3.60 Billion
2024
2032
| 2025 –2032 | |
| USD 1.80 Billion | |
| USD 3.60 Billion | |
|
|
|
|
North America Biopsy Devices Market Segmentation, By Product (Needle-Based Biopsy Instruments, Procedure Trays, Localization Wires and other Products), Application (Breast Biopsy, Lung Biopsy, Colorectal Biopsy, Prostate Biopsy and Other Applications), Guidance Technique (Ultrasound-Guided Biopsy, Stereotactic-Guided Biopsy, MRI-Guided Biopsy and Other Guidance Techniques), End User (Hospitals, Academic and Research Institutes, Diagnostic and Imaging Centers) – Industry Trends and Forecast to 2032
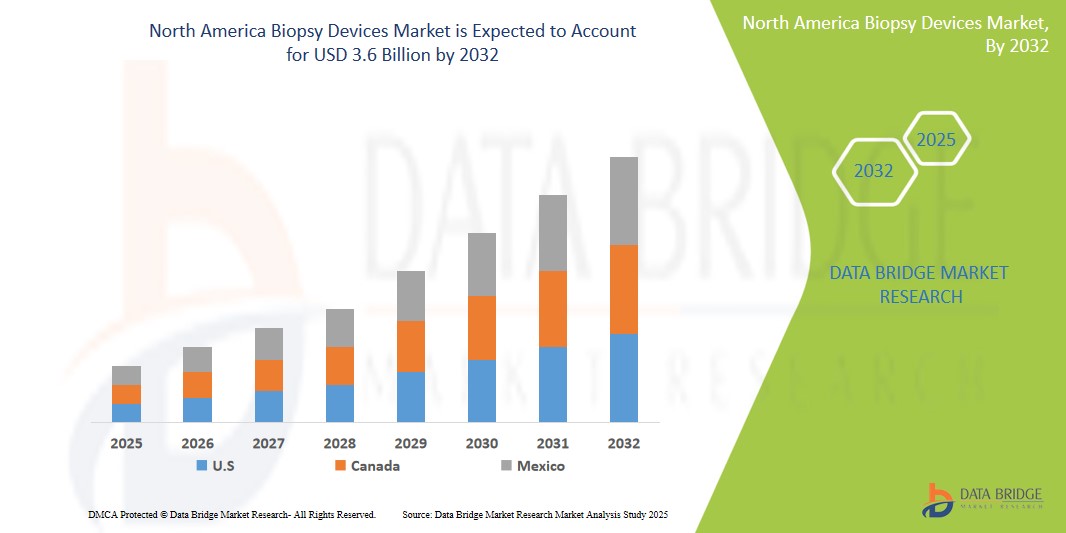
North America Biopsy Devices Market Size
- The North America Biopsy Devices Market was valued atUSD1.8 Billion in 2024 and is expected to reachUSD3.6 Billion by 2032
- Drivers of the North America Biopsy Devices Market include the rising prevalence of cancer and other chronic diseases, which necessitate accurate diagnostic tools for early detection and treatment. Additionally, advancements in biopsy technologies, such as minimally invasive techniques and imaging guidance, are enhancing procedural efficiency and patient outcomes. The increasing emphasis on personalized medicine and the growing number of diagnostic and outpatient procedures further propel market growth.
North America Biopsy Devices Market Analysis
- The North America Biopsy Devices Market is experiencing robust growth due to the rising incidence of cancer and an increasing focus on early diagnosis and intervention. The aging population and a greater awareness of health issues contribute to the demand for advanced biopsy technologies.
- Innovations in biopsy techniques, including image-guided biopsies and minimally invasive procedures, are driving the market. These advancements improve accuracy, reduce patient discomfort, and shorten recovery times, making them more appealing to healthcare providers and patients alike.
- Favorable regulatory environments and reimbursement policies in the U.S. and Canada support the adoption of biopsy devices. This environment encourages manufacturers to develop and market advanced diagnostic tools, further boosting market growth.
- The market features several major players, including companies like Bard, Hologic, and Devicor Medical Products, which are continually innovating and expanding their product offerings. Competitive strategies often involve mergers, acquisitions, and partnerships to enhance market presence and technological capabilities.
Report Scope andNorth America Biopsy Devices MarketSegmentation
|
Attributes |
North America Biopsy Devices MarketInsights |
|
Segments Covered |
|
|
Countries Covered |
North America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
North America Biopsy Devices MarketTrends
“Growing Adoption of Image-Guided Biopsy Techniques”
- Image-guided biopsy techniques, such as ultrasound, CT, and MRI-guided procedures, enhance the accuracy of sample collection, allowing for more precise targeting of lesions. This precision is crucial for effective diagnosis, particularly in complex cases, leading to better patient outcomes.
- Patients and healthcare providers increasingly favor minimally invasive techniques that reduce recovery time and postoperative complications. Image-guided biopsies fulfill this demand, offering safer alternatives to traditional surgical biopsies with shorter hospital stays and quicker return to daily activities.
- Continuous advancements in imaging technologies are facilitating the integration of high-resolution imaging with biopsy devices, enabling more effective and streamlined procedures. As these technologies evolve, their application in biopsy devices is expected to become even more widespread, driving market growth and innovation.
North America Biopsy Devices Market Dynamics
Driver
“Increasing Incidence of Cancer”
- The incidence of various types of cancer, including breast, lung, and prostate cancer, is increasing in North America due to factors such as an aging population, lifestyle changes, and environmental exposures. This rise in cancer cases boosts the demand for diagnostic devices, including biopsy devices, which are essential for accurate diagnosis and treatment planning.
- Early and accurate detection of cancer significantly improves treatment outcomes and increases survival rates. As healthcare providers emphasize preventive care and early diagnosis, the demand for biopsy devices that facilitate timely and effective cancer detection has surged, driving market growth.
- Continuous innovation in biopsy technology, including the development of less invasive and more precise methods, enhances the appeal of biopsy devices. These advancements not only improve diagnostic capabilities but also encourage healthcare providers to adopt biopsies as a standard practice in cancer diagnosis, further fueling market demand.
Opportunity
“Expanding Applications of Biopsy Devices in Oncology”
- The rising prevalence of various cancers, particularly breast, lung, and colorectal cancers, presents a significant opportunity for the biopsy devices market. Early and accurate diagnostic techniques, including biopsies, are essential for effective treatment planning and management, leading to greater demand for advanced biopsy solutions.
- As the focus on personalized medicine grows, there is an increasing need for precise tumor characterization through biopsy techniques. This enables tailored treatments based on the specific genetic and molecular profile of a patient’s cancer, expanding the role of biopsy devices in oncology and enhancing their market potential.
- The ongoing development of novel biopsy technologies, such as liquid biopsies and advanced imaging-guided procedures, offers new avenues for market growth. These innovations provide less invasive options for obtaining tissue samples and enhance diagnostic accuracy, making biopsy devices more appealing to both providers and patients.
Restraint/Challenge
“Regulatory and Reimbursement Issues”
- The process for obtaining regulatory approval for new biopsy devices in North America can be lengthy and complex. Manufacturers must navigate through rigorous standards set by organizations like the FDA, which can delay the introduction of innovative products to the market and increase development costs.
- Securing reimbursement from insurance providers for biopsy procedures can be challenging. Variability in coverage policies and reimbursement rates across different insurers can deter healthcare providers from adopting newer biopsy technologies, limiting their market potential.
- High costs associated with advanced biopsy devices may restrict their accessibility, especially in lower-resource healthcare settings. Hospitals and clinics may be hesitant to invest in expensive technologies without assurance of adequate reimbursement, further complicating market penetration.
North America Biopsy Devices Market Scope
The market is segmented on the basis of type, end user, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Product |
Needle-Based Biopsy Instruments, Procedure Trays, Localization Wires and other Products |
|
ByApplication |
Breast Biopsy, Lung Biopsy, Colorectal Biopsy, Prostate Biopsy and Other Applications |
|
ByGuidance Technique |
Ultrasound-Guided Biopsy, Stereotactic-Guided Biopsy, MRI-Guided Biopsy and Other Guidance Techniques |
|
By End User |
Hospitals, Academic and Research Institutes, Diagnostic and Imaging Centers) |
North America Biopsy Devices Market Analysis
“U.S. is the Dominant with around 38% market share in the North America Biopsy Devices Market”
- The United States boasts a highly developed healthcare system with sophisticated medical facilities and advanced technological capabilities. This infrastructure supports the adoption of cutting-edge biopsy devices, making the U.S. a leader in the market. Hospitals and clinics in the U.S. are equipped with the latest imaging technologies and biopsy tools, which enhances the precision and efficiency of diagnostic procedures.
- The U.S. is home to a large number of medical device manufacturers and biotech companies that heavily invest in research and development. This commitment leads to continuous innovation in biopsy technology, including the development of minimally invasive techniques and advanced imaging-guided devices. The financial resources available for R&D contribute to the growth and dominance of the U.S. in the biopsy market.
- The U.S. healthcare system offers a relatively robust reimbursement framework for biopsy procedures, facilitating greater access to these services for patients. Insurance companies and government programs often cover advanced diagnostic procedures, encouraging healthcare providers to adopt the latest biopsy technologies. This support creates a conducive environment for market expansion and places the U.S. at the forefront of the North American Biopsy Devices Market.
“U.S. is Projected to Register the Highest Growth Ratein theNorth America Biopsy Devices Market”
- The U.S. is home to a robust ecosystem of research and development in the healthcare sector, leading to significant innovations in biopsy devices. Continuous advancements in imaging technologies and minimally invasive techniques are driving the adoption of sophisticated biopsy solutions, thus fueling market growth.
- The rising prevalence of cancer and other chronic diseases in the U.S. has led to a higher demand for diagnostic procedures, including biopsies. As early detection of cancer becomes a priority in healthcare, the need for advanced biopsy devices is expected to rise, contributing to the market’s growth in the region.
North America Biopsy Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- BD (Becton, Dickinson and Company)
- Hologic, Inc.
- Danaher Corporation ( (including brands like Leica Biosystems)
- Argon Medical Devices (
- Cook Medical
- AbbVie Inc. (Allergan)
- Bayer AGss
- The Cooper Companies, Inc.
- DKT International
- EUROGINE S.L.
- Pregna International Limited
- Prosan International BV
- SMB Corporation of India
- Melbea AG
- OCON Medical Ltd.
- Mylan N.V. (Viatris)
- Teva Pharmaceutical Industries Ltd.
- Merck & Co., Inc.
- Pfizer Inc.
- HLL Lifecare Limited
Latest Developments in North America Biopsy Devices Market
- In 2024, Hologic, Inc. launched its Breast Biopsy Suite, integrating advanced imaging and biopsy tools for more precise diagnostics.
- In 2023, BD (Becton, Dickinson and Company) introduced a next-generation core biopsy needle with enhanced tissue sample accuracy.
- In 2023, Argon Medical Devices released a new vacuum-assisted biopsy system for minimally invasive procedures.
SKU-
Erhalten Sie Online-Zugriff auf den Bericht zur weltweit ersten Market Intelligence Cloud
- Interaktives Datenanalyse-Dashboard
- Unternehmensanalyse-Dashboard für Chancen mit hohem Wachstumspotenzial
- Zugriff für Research-Analysten für Anpassungen und Abfragen
- Konkurrenzanalyse mit interaktivem Dashboard
- Aktuelle Nachrichten, Updates und Trendanalyse
- Nutzen Sie die Leistungsfähigkeit der Benchmark-Analyse für eine umfassende Konkurrenzverfolgung
Forschungsmethodik
Die Datenerfassung und Basisjahresanalyse werden mithilfe von Datenerfassungsmodulen mit großen Stichprobengrößen durchgeführt. Die Phase umfasst das Erhalten von Marktinformationen oder verwandten Daten aus verschiedenen Quellen und Strategien. Sie umfasst die Prüfung und Planung aller aus der Vergangenheit im Voraus erfassten Daten. Sie umfasst auch die Prüfung von Informationsinkonsistenzen, die in verschiedenen Informationsquellen auftreten. Die Marktdaten werden mithilfe von marktstatistischen und kohärenten Modellen analysiert und geschätzt. Darüber hinaus sind Marktanteilsanalyse und Schlüsseltrendanalyse die wichtigsten Erfolgsfaktoren im Marktbericht. Um mehr zu erfahren, fordern Sie bitte einen Analystenanruf an oder geben Sie Ihre Anfrage ein.
Die wichtigste Forschungsmethodik, die vom DBMR-Forschungsteam verwendet wird, ist die Datentriangulation, die Data Mining, die Analyse der Auswirkungen von Datenvariablen auf den Markt und die primäre (Branchenexperten-)Validierung umfasst. Zu den Datenmodellen gehören ein Lieferantenpositionierungsraster, eine Marktzeitlinienanalyse, ein Marktüberblick und -leitfaden, ein Firmenpositionierungsraster, eine Patentanalyse, eine Preisanalyse, eine Firmenmarktanteilsanalyse, Messstandards, eine globale versus eine regionale und Lieferantenanteilsanalyse. Um mehr über die Forschungsmethodik zu erfahren, senden Sie eine Anfrage an unsere Branchenexperten.
Anpassung möglich
Data Bridge Market Research ist ein führendes Unternehmen in der fortgeschrittenen formativen Forschung. Wir sind stolz darauf, unseren bestehenden und neuen Kunden Daten und Analysen zu bieten, die zu ihren Zielen passen. Der Bericht kann angepasst werden, um Preistrendanalysen von Zielmarken, Marktverständnis für zusätzliche Länder (fordern Sie die Länderliste an), Daten zu klinischen Studienergebnissen, Literaturübersicht, Analysen des Marktes für aufgearbeitete Produkte und Produktbasis einzuschließen. Marktanalysen von Zielkonkurrenten können von technologiebasierten Analysen bis hin zu Marktportfoliostrategien analysiert werden. Wir können so viele Wettbewerber hinzufügen, wie Sie Daten in dem von Ihnen gewünschten Format und Datenstil benötigen. Unser Analystenteam kann Ihnen auch Daten in groben Excel-Rohdateien und Pivot-Tabellen (Fact Book) bereitstellen oder Sie bei der Erstellung von Präsentationen aus den im Bericht verfügbaren Datensätzen unterstützen.