Asia Pacific Dry Eye Syndrome Treatment Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
1.04 Billion
USD
2.12 Billion
2024
2032
USD
1.04 Billion
USD
2.12 Billion
2024
2032
| 2025 –2032 | |
| USD 1.04 Billion | |
| USD 2.12 Billion | |
|
|
|
|
Asia-Pacific Dry Eye Syndrome Treatment Market, By Drug Class (Anti-inflammatory Drugs, Artificial Tears, Secretagogues, Autologous Serum Eye Drops, Others), Route of Administration (Topical, Oral, Others), End-Users (Hospitals, Ophthalmic Clinics, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy) – Industry Trends and Forecast to 2032
Dry Eye Syndrome Treatment Market Size
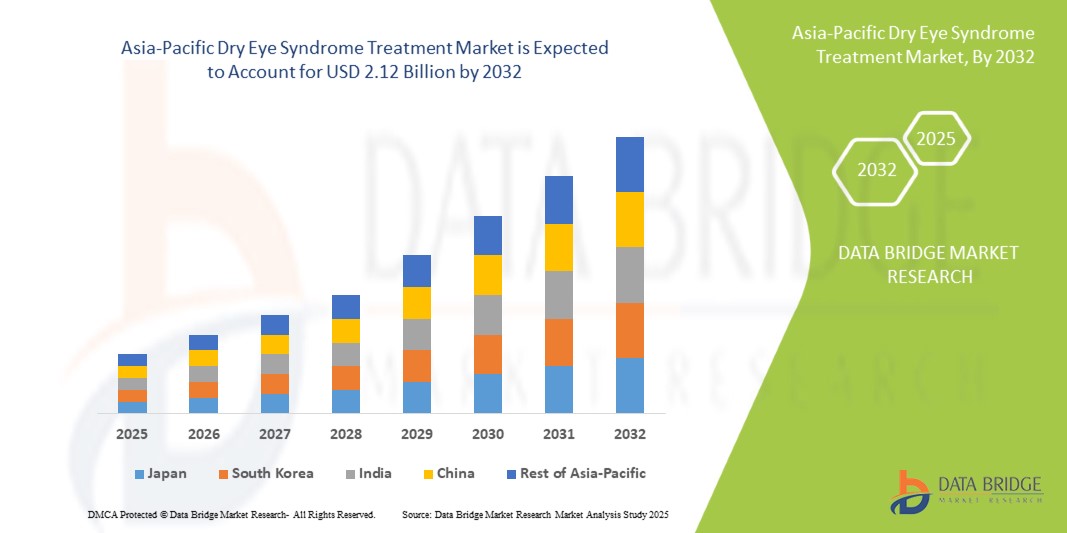
- The Asia-Pacific Dry Eye Syndrome Treatment Market was valued at USD 1.04 billion in 2024 and is expected to reach USD 2.12 billion by 2032.
- During the forecast period of 2025 to 2032, the market is projected to grow at a CAGR of 9.1%, primarily driven by the increasing prevalence of dry eye associated with screen exposure, environmental pollution, and aging demographics.
- This growth is fueled by factors such as a surge in digital device usage, growing awareness about ocular surface disorders, and advancements in tear film diagnostics and therapies.
Dry Eye Syndrome Treatment Market Analysis
- The Asia-Pacific Dry Eye Syndrome Treatment Market is anticipated to experience robust growth during the forecast period, with a CAGR of 9.1% from 2025 to 2032.
- Dry Eye Syndrome (DES), also known as keratoconjunctivitis sicca, is a multifactorial disease affecting the tear film and ocular surface, commonly caused by meibomian gland dysfunction, environmental factors, and systemic medications.
- The market is being driven by a rising demand for preservative-free artificial tears, development of novel anti-inflammatory eye drops, and incorporation of regenerative medicine such as autologous serum-based treatments.
- Factors such as increasing healthcare infrastructure, improved accessibility to specialized eye care, and a rise in awareness campaigns across emerging countries like India, China, and Southeast Asia are expected to support market expansion
Report Scope andDry Eye Syndrome Treatment Market Segmentation
|
Attributes |
Dry Eye Syndrome Treatment KeyMarket Insights |
|
Segments Covered |
|
|
Countries Covered |
Asia-Pacific
|
|
Key Market Players |
|
|
Market Opportunities |
Growing healthcare facilities Rise in the prevalence of delirium |
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
- Asia-Pacific Dry Eye Syndrome Treatment Market Trends
“Shift Toward Biologic and Targeted Immunomodulatory Therapies”
A key trend in the Asia-Pacific Dry Eye Syndrome Treatment market is the growing adoption of biologic agents and targeted therapies, particularly for cases associated with autoimmune conditions and chronic inflammatory diseases that are unresponsive to conventional corticosteroids. The region is increasingly focusing on TNF-alpha inhibitors, IL-6 antagonists, and T-cell modulators to manage inflammation at the molecular level with higher precision.
- For instance, adalimumab, a TNF-alpha inhibitor, has seen expanded use in several Asia-Pacific countries for non-infectious intermediate and posterior uveitis, offering prolonged remission and fewer relapses.
- The integration of optical coherence tomography (OCT), AI-based diagnostic tools, and personalized medicine platforms is optimizing disease monitoring and therapeutic decisions.
Additionally, advancements in long-acting ocular drug delivery systems, including depot formulations and intraocular implants, are significantly enhancing patient adherence and minimizing systemic adverse effects.
Asia-Pacific Dry Eye Syndrome Treatment Market Dynamics
Driver
“Growing Prevalence of Autoimmune and Inflammatory Eye Diseases”
The incidence of inflammatory eye disorders is on the rise in Asia-Pacific due to increasing autoimmune diseases and environmental triggers that affect ocular health.
For instance:
- According to the Asia-Pacific Academy of Ophthalmology (2023), inflammatory uveitis contributes significantly to visual impairment in the region, with autoimmune diseases such as Behçet’s disease and VKH syndrome being prevalent causes.
- The chronic nature and risk of vision loss associated with these conditions are driving demand for long-term, steroid-sparing therapies that provide both efficacy and safety.
“Regulatory Advancements and Fast-Track Designations for Biologics”
Progressive regulatory initiatives and increased funding for biologic drug development are accelerating the introduction of novel treatments for uveitic and dry eye conditions in Asia-Pacific.
For example:
- In countries like Japan, South Korea, and Australia, regulatory agencies have granted priority review and orphan drug status to biologics addressing non-infectious uveitis, enabling quicker market entry and improved treatment availability.
Opportunity
“Pipeline Expansion and Partnerships for Innovative Drug Development”
The Asia-Pacific Dry Eye Syndrome Treatment market is experiencing strong growth due to increased R&D, cross-border collaborations, and investments in novel therapeutic mechanisms.
For example:
- Regional pharmaceutical companies are investigating kinase inhibitors, S1P receptor modulators, and microbiome-derived therapies as next-generation treatments.
- Collaborations between universities, biotech firms, and government agencies are fostering innovation, particularly in emerging markets like India and China, where unmet needs remain high.
- The push for personalized medicine and development of first-in-class treatments is creating vast opportunities across diversified patient segments.
Restraint/Challenge
“Cost Constraints and Variability in Treatment Outcomes”
Despite technological advancements, the high cost of biologics and targeted therapies remains a significant barrier in several Asia-Pacific markets, particularly in developing countries.Access to sophisticated diagnostic tools and advanced medications is still limited across rural and underserved areas, affecting equitable treatment delivery.Patient response variability, driven by genetic diversity, environmental exposures, and microbiome composition, adds complexity to achieving uniform therapeutic success.
For example:
- tofacitinib has demonstrated clinical efficacy in autoimmune-related eye conditions, its high cost, risk of serious side effects, and the need for frequent monitoring hinder its broader use in routine care across the Asia-Pacific region
Dry Eye Syndrome Treatment market Scope
The market is segmented on the basis of type, drug class, application, dosage, route of administration, end-users, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
Drug Class |
|
|
Route of Administration |
|
|
End-Users |
|
|
Distribution Channel |
|
Asia Pacific Dry Eye Syndrome Treatment Market Regional Analysis
“North America is the Dominant Region in the Dry Eye Syndrome Treatment market”
- North America dominates the Asia Pacific Dry Eye Syndrome Treatment market owing to its advanced pharmaceutical ecosystem, robust healthcare infrastructure, and high awareness of ocular health and uveitis management.
- The United States holds the largest market share in this region, driven by a high incidence of uveitis, availability of specialized ophthalmologists, and rising cases of autoimmune conditions linked with uveitis.
- The region benefits from substantial R&D funding, presence of major drug manufacturers, and a strong regulatory environment led by the JAPAN. Food and Drug Administration (FDA), which ensures drug safety and efficacy.
- Increasing demand for biologics, corticosteroids, and immunosuppressive therapies along with rising adoption of advanced diagnostic tools contributes to continued market expansion.
- Collaborations between academic research institutions and pharmaceutical giants, alongside clinical advancements and innovation in drug delivery systems, are fostering growth in this region.
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- The Asia-Pacific region is expected to exhibit the fastest growth in the Dry Eye Syndrome Treatment market, owing to increasing healthcare investments, a growing patient population, and improving access to specialty care.
- Key countries such as India, China, Japan, and South Korea are leading this growth, supported by rising awareness of uveitis, increasing prevalence of infectious and non-infectious forms of the disease, and improved ophthalmology services.
- Supportive government policies to enhance domestic pharmaceutical production and reduce reliance on imports are accelerating regional development.
- Japan, with its aging population and high healthcare standards, has shown strong adoption of advanced uveitis treatments and contributes significantly to regional market progress.
- A surge in clinical trials, expansion of biosimilar manufacturing, and collaborations between Asia Pacific pharmaceutical leaders and local players are expected to drive long-term growth across Asia-Pacific.
Dry Eye Syndrome Treatment Market Share
The competitive landscape provides comprehensive insights into key market players, detailing their profiles, financial data, R&D efforts, product portfolios, operational footprint, manufacturing capacity, strategic initiatives, strengths, weaknesses, and contributions to the uveitis treatment segment.
The Major Market Leaders Operating in the Market Include:
- AbbVie Inc.
- Novartis AG
- Bausch + Lomb
- Johnson & Johnson Services, Inc.
- Allergan (a subsidiary of AbbVie)
- Santen Pharmaceutical Co., Ltd.
- Eyegate Pharmaceuticals, Inc.
- EyePoint Pharmaceuticals, Inc.
- Alimera Sciences
- Clearside Biomedical, Inc.
- Teva Pharmaceutical Industries Ltd.
- Sun Pharmaceutical Industries Ltd.
- Pfizer Inc.
- Roche Holding AG
- Regeneron Pharmaceuticals, Inc.
- Amgen Inc.
- Acelyrin, Inc.
- MeiraGTx Holdings plc
- Ocugen, Inc.
- Aldeyra Therapeutics, Inc.
Latest Developments in Asia Pacific Dry Eye Syndrome Treatment Market
- In March 2022, a report from the International Agency for the Prevention of Blindness outlined significant challenges in the Dry Eye Syndrome Treatmentsector, including gaps in early diagnosis, high treatment costs, and the complexity of managing chronic and recurrent forms of the disease.
- The report also emphasized the growing importance of green chemistry, continuous manufacturing technologies, and regional self-reliance in reducing supply disruptions. Increased industry focus on API traceability and quality assurance is driving a shift toward more sustainable and transparent production practices in gastrointestinal therapeutics
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.