Mercado de subcontratación de asuntos regulatorios de IVD de Asia y el Pacífico, por servicio (redacción y presentaciones regulatorias, registro regulatorio y solicitudes de ensayos clínicos, consultoría regulatoria, representación legal, servicios de gestión de datos, servicios de fabricación y controles químicos (CMC) y otros), indicación (oncología, neurología , cardiología, química clínica e inmunoensayos, medicina de precisión , enfermedades infecciosas, diabetes, pruebas genéticas, VIH/SIDA, hematología, pruebas de medicamentos/farmacogenómica, transfusión de sangre, punto de atención y otros), modelo de implementación (nube y en las instalaciones), tamaño de la organización (pequeñas y medianas empresas (PYME) y grandes empresas), etapa (clínica, preclínica y PMA (autorización posterior a la comercialización)), clase (clase I, clase II y clase III), usuario final (compañías farmacéuticas, compañías de dispositivos médicos, compañías de biotecnología y otras), país (China, Corea del Sur, Japón, India, Australia, Singapur, Malasia, Indonesia, Tailandia, Filipinas y resto de Asia-Pacífico) Tendencias de la industria y pronóstico hasta 2029.
Análisis y perspectivas del mercado : mercado de subcontratación de asuntos regulatorios de diagnóstico in vitro en Asia y el Pacífico
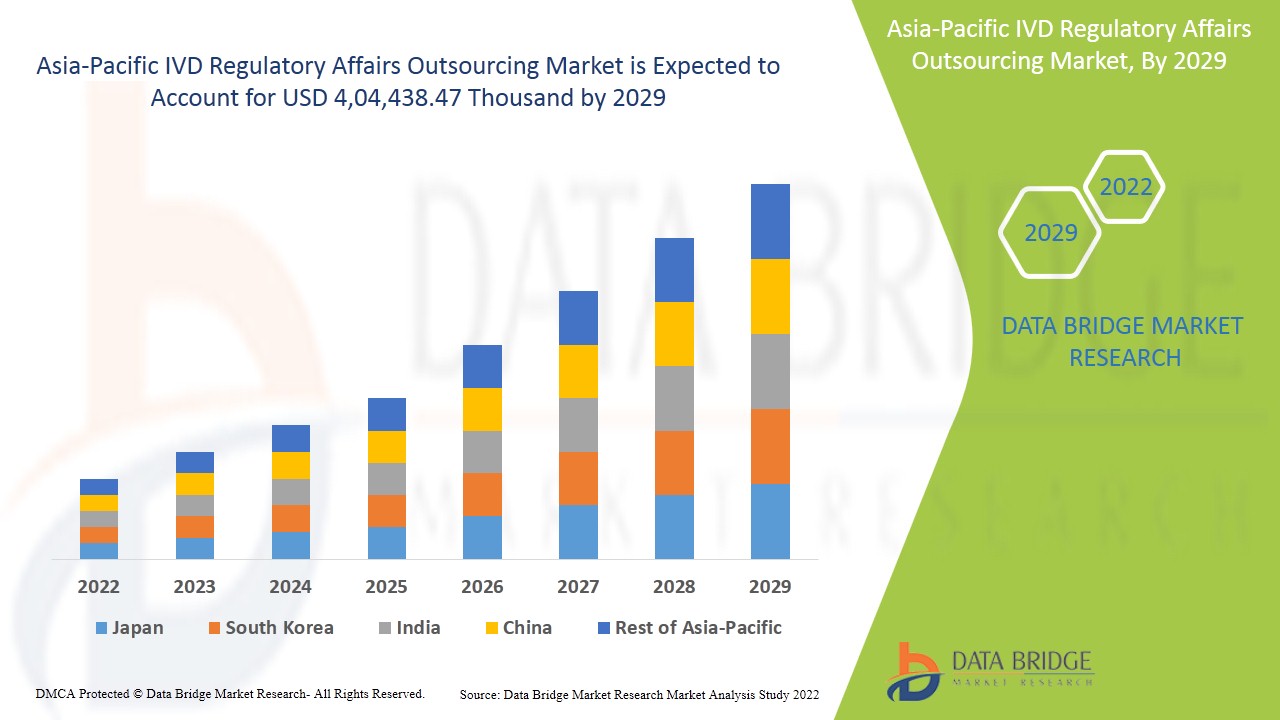
Se espera que el mercado de subcontratación de asuntos regulatorios de IVD de Asia-Pacífico gane crecimiento de mercado en el período de pronóstico de 2022 a 2029. Data Bridge Market Research analiza que el mercado está creciendo con una CAGR del 14,1% en el período de pronóstico de 2022 a 2029 y se espera que alcance los USD 4, 04,438.47 mil para 2029.
- Los productos de diagnóstico in vitro son reactivos, dispositivos y sistemas que se utilizan para diagnosticar enfermedades u otras afecciones, incluida la determinación del estado de salud de una persona para curar, mitigar, tratar o prevenir enfermedades. Estos productos están destinados a su uso en la recolección, preparación y examen de muestras del cuerpo humano. Los asuntos regulatorios desempeñan un papel crucial en la industria de dispositivos de diagnóstico in vitro (IVD) y dispositivos médicos. Los servicios de subcontratación de asuntos regulatorios implican la redacción médica y la publicación de documentación regulatoria por parte de profesionales que contribuyen a la producción de documentos de alta calidad para proyectos de investigación clínica. La demanda de subcontratación de servicios regulatorios está aumentando sustancialmente en los estudios clínicos realizados en economías emergentes, lo que proporciona una plataforma saludable para el crecimiento de esta industria.
Los principales factores que impulsan el crecimiento del mercado de subcontratación de asuntos regulatorios de IVD son el aumento de la prevalencia de enfermedades crónicas en toda la región y el avance tecnológico en varios dispositivos de diagnóstico in vitro. El aumento de la adquisición estratégica y la asociación entre organizaciones está creando oportunidades para el crecimiento del mercado. El cambio de las regulaciones con respecto a los dispositivos médicos en diferentes regiones está actuando como la principal restricción para el mercado de subcontratación de asuntos regulatorios de IVD. La falta de infraestructura en el servicio de atención médica está actuando como un desafío importante para el crecimiento del mercado.
Este informe de mercado sobre la subcontratación de asuntos regulatorios de IVD proporciona detalles sobre la participación de mercado, los nuevos desarrollos y el análisis de la cartera de productos, el impacto de los actores del mercado local y nacional, analiza las oportunidades en términos de nuevos segmentos de ingresos, cambios en las regulaciones del mercado, aprobaciones de productos, decisiones estratégicas, lanzamientos de productos, expansiones geográficas e innovaciones tecnológicas en el mercado. Para comprender el análisis y el escenario del mercado, comuníquese con nosotros para obtener un resumen analítico; nuestro equipo lo ayudará a crear una solución de impacto en los ingresos para lograr su objetivo deseado.
Alcance y tamaño del mercado de subcontratación de asuntos regulatorios de diagnóstico in vitro en Asia y el Pacífico
El mercado de subcontratación de asuntos regulatorios de IVD de Asia-Pacífico está segmentado en siete segmentos notables que se basan en los servicios, la indicación, el modo de implementación, el tamaño de la organización, la etapa, la clase y el usuario final.
- En términos de servicios, el mercado de subcontratación de asuntos regulatorios de diagnóstico in vitro de Asia-Pacífico se segmenta en redacción y presentación de documentos regulatorios, registro regulatorio y solicitudes de ensayos clínicos, consultoría regulatoria, representación legal, servicios de gestión de datos, servicios de fabricación y control de productos químicos (CMC) y otros. En 2022, se espera que la redacción y presentación de documentos regulatorios domine el mercado a medida que aumenten los números de registro de productos y las aprobaciones de ensayos clínicos en toda la región.
- Según las indicaciones, el mercado de subcontratación de asuntos regulatorios de IVD en Asia-Pacífico está segmentado en oncología, neurología, cardiología, química clínica e inmunoensayos, medicina de precisión, enfermedades infecciosas, diabetes, pruebas genéticas, VIH/SIDA, hematología, pruebas de drogas/farmacogenómica, transfusión de sangre, punto de atención y otros. En 2022, se espera que el segmento de oncología domine a medida que la cartera de servicios y tecnología de alta calidad relacionada con la aplicación regulatoria de IVD atraiga una mayor participación de los pares.
- En función del modo de implementación, el mercado de subcontratación de asuntos regulatorios de IVD de Asia-Pacífico se segmenta en la nube y en las instalaciones. En 2022, se espera que el segmento de la nube domine, ya que es rentable y las características flexibles de la solución de la tecnología de computación en la nube en la regulación de IVD brindan una infraestructura estable con el máximo rendimiento para las organizaciones que se ocupan de sistemas de IVD.
- En función del tamaño de la organización, el mercado de subcontratación de asuntos regulatorios de diagnóstico in vitro de Asia-Pacífico se segmenta en pequeñas y medianas empresas (PYMES) y grandes empresas. En 2022, se espera que el segmento de las grandes empresas domine, ya que implica la representación legal del producto y, debido a las estrictas regulaciones que pueden variar de un país a otro, implica un alto consumo de recursos debido a la gran demanda de recursos y a los cambios en las políticas.
- En función de la etapa, el mercado de subcontratación de asuntos regulatorios de IVD de Asia-Pacífico se segmenta en clínico, preclínico y PMA (autorización posterior a la comercialización). En 2022, se espera que el segmento clínico domine el mercado; los actores de los dispositivos IVD en el mercado deben seguir ciertas regulaciones para obtener la aprobación de las autoridades superiores para el lanzamiento del producto en una región. Estas estrictas pautas deben seguirse y esta es una de las tareas más difíciles entre todos los pasos. La aprobación previa a la comercialización de varios dispositivos médicos varía de un país a otro.
- En función de la clase, el mercado de subcontratación de asuntos regulatorios de diagnóstico in vitro de Asia-Pacífico se segmenta en clase I, clase II y clase III. En 2022, se espera que el segmento de clase I domine el mercado, ya que involucra el 47 % de los dispositivos médicos y no presenta riesgo para la salud pública o presenta un riesgo personal bajo con las regulaciones más bajas.
- En función del usuario final, el mercado de subcontratación de asuntos regulatorios de diagnóstico in vitro de Asia-Pacífico se segmenta en empresas farmacéuticas, empresas de dispositivos médicos, empresas de biotecnología y otras. En 2022, se espera que las empresas de dispositivos médicos participen en I+D en toda la región debido a la demanda de normativas de diagnóstico in vitro.
Análisis a nivel de país del mercado de subcontratación de asuntos regulatorios de diagnóstico in vitro en Asia y el Pacífico
Se analiza el mercado de subcontratación de asuntos regulatorios de IVD de Asia y el Pacífico y se proporciona información sobre el tamaño del mercado por país, servicios, indicación, modo de implementación, tamaño de la organización, etapa, clase y usuario final.
Los países cubiertos en el informe del mercado de subcontratación de asuntos regulatorios de IVD de Asia-Pacífico son China, Corea del Sur, Japón, India, Australia, Singapur, Malasia, Indonesia, Tailandia, Filipinas y el resto de Asia-Pacífico.
Se espera que China domine el mercado en la región Asia-Pacífico debido a los crecientes avances en el sector de la salud y la disponibilidad del laboratorio clínico más grande de Asia-Pacífico.
La sección de países del informe también proporciona factores de impacto de mercado individuales y cambios en la regulación en el mercado a nivel nacional que afectan las tendencias actuales y futuras del mercado. Los puntos de datos como nuevas ventas, ventas de reemplazo, demografía del país, leyes regulatorias y aranceles de importación y exportación son algunos de los principales indicadores utilizados para pronosticar el escenario del mercado para países individuales. Además, la presencia y disponibilidad de marcas globales y sus desafíos enfrentados debido a la competencia grande o escasa de las marcas locales y nacionales, el impacto de los canales de venta se consideran al proporcionar un análisis de pronóstico de los datos del país.
Demanda creciente de subcontratación de asuntos regulatorios de diagnóstico in vitro
El mercado de subcontratación de asuntos regulatorios de IVD de Asia-Pacífico también le proporciona un análisis detallado del mercado para el crecimiento de la industria de cada país con ventas, ventas de componentes, impacto del desarrollo tecnológico en la subcontratación de asuntos regulatorios de IVD y cambios en los escenarios regulatorios con su apoyo al mercado de subcontratación de asuntos regulatorios de IVD. Los datos están disponibles para el período histórico de 2011 a 2019.
Análisis del panorama competitivo y de la cuota de mercado de subcontratación de asuntos regulatorios de diagnóstico in vitro en Asia y el Pacífico
El panorama competitivo del mercado de externalización de asuntos regulatorios de diagnóstico in vitro de Asia y el Pacífico proporciona detalles por competidor. Los detalles incluidos son una descripción general de la empresa, las finanzas de la empresa, los ingresos generados, el potencial de mercado, la inversión en investigación y desarrollo, las nuevas iniciativas de mercado, la presencia global, los sitios e instalaciones de producción, las fortalezas y debilidades de la empresa, el lanzamiento de productos, las líneas de prueba de productos, las aprobaciones de productos, las patentes, la amplitud y la extensión de los productos, el dominio de las aplicaciones, la curva de la línea de vida de la tecnología. Los puntos de datos anteriores proporcionados solo están relacionados con el enfoque de las empresas en relación con el mercado de externalización de asuntos regulatorios de diagnóstico in vitro de Asia y el Pacífico.
Algunos de los principales actores que operan en el mercado de subcontratación de asuntos regulatorios de IVD son Freyr Solutions, PPD Inc. (una subsidiaria de Thremofisher Scientific Inc.), EMERGO, ICON, Parexel International Corporation, CRITERIUM, INC., Groupe ProductLife SA, Labcorp Drug Development, WuXi AppTec, Genpact, Medpace, Dor Pharmaceutical Services, Qserve, entre otros. Los analistas de DBMR comprenden las fortalezas competitivas y brindan un análisis competitivo para cada competidor por separado.
Muchas empresas de todo el mundo también están iniciando desarrollos de productos que también están acelerando el crecimiento del mercado de subcontratación de asuntos regulatorios de IVD en Asia-Pacífico.
Por ejemplo,
- En noviembre de 2021, la revista tecnológica USA-9 incluyó a Freyr en la lista de los «10 mejores proveedores de soluciones tecnológicas de 2021». USA-9.com es una revista tecnológica que ha incluido a Freyr Solutions, un proveedor líder mundial de soluciones y servicios regulatorios, como uno de los «10 mejores proveedores de soluciones tecnológicas de 2021», ya que Fryer continúa diseñando soluciones de software innovadoras y apoyando a los clientes en sus respectivos objetivos de cumplimiento. Esto ha ayudado a la empresa a aumentar su popularidad.
- En octubre de 2021, el grupo Propharma adquirió Pharmica Consulting. ProPharma Group, una empresa de cartera de Odyssey Investment Partners, ha adquirido Pharmica Consulting, una empresa de consultoría de ciencias biológicas que ofrece soluciones de consultoría de gestión de proyectos (PM) y software de operaciones patentado a empresas farmacéuticas y biotecnológicas para la ejecución de ensayos clínicos. Esto ha ayudado a la empresa a expandir su negocio a nivel mundial en el mercado.
Las asociaciones, las empresas conjuntas y otras estrategias mejoran la participación de mercado de la empresa con una mayor cobertura y presencia. También brindan a las organizaciones la ventaja de mejorar su oferta de subcontratación de asuntos regulatorios de diagnóstico in vitro mediante una gama más amplia de tamaños.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 DBMR MARKET POSITION GRID
2.7 VENDOR SHARE ANALYSIS
2.8 MULTIVARIATE MODELING
2.9 SERVICE TIMELINE CURVE
2.1 MARKET END USER COVERAGE GRID
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, REGULATORY SCENARIO
4.1.1 THE U.S.
4.1.2 REGULATIONS IN EUROPE
4.1.3 REGULATIONS IN ASIA
4.1.3.1 CHINA
4.1.3.2 SOUTH KOREA
4.1.3.3 MALAYSIA
4.1.3.4 THAILAND
5 MARKET OVERVIEW
5.1 DRIVERS
5.1.1 RISE IN PREVALENCE OF CHRONIC DISEASES ACROSS THE REGION
5.1.2 TECHNOLOGICAL ADVANCEMENT IN DEVELOPING VARIOUS IN VITRO DIAGNOSTIC DEVICES
5.1.3 DEVELOPMENT OF PROJECT-BASED SUPPORT LEADS TO LONG TERM OUTSOURCING AGREEMENT AMONG ORGANIZATION
5.1.4 INCREASE IN PRODUCT REGISTRATION NUMBERS AND CLINICAL TRIAL APPROVALS ACROSS THE REGION
5.2 RESTRAINTS
5.2.1 STRINGENT REGULATIONS REGARDING MEDICAL DEVICES IN DIFFERENT REGIONS
5.2.2 HIGHER COST RELATED TO MAINTENANCE AND OUTSOURCING OF IVD
5.3 OPPORTUNITIES
5.3.1 RISE IN STRATEGIC ACQUISITION & PARTNERSHIP AMONG ORGANIZATION
5.3.2 EMERGENCE OF VARIOUS EFFICIENT TECHNOLOGICAL SERVICES AND STANDARDS
5.3.3 INCREASE IN R&D ACTIVITIES BY COMPANIES ACROSS THE REGION
5.4 CHALLENGES
5.4.1 LACK OF INFRASTRUCTURE IN HEALTHCARE SERVICE
5.4.2 SHORTAGE OF SKILLED PERSONNEL FOR HANDLING IN VITRO DIAGNOSTIC DEVICES
6 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE
6.1 OVERVIEW
6.2 REGULATORY WRITING & SUBMISSIONS
6.3 LEGAL REPRESENTATION
6.4 REGULATORY CONSULTING
6.5 REGULATORY REGISTRATION & CLINICAL TRIAL APPLICATIONS
6.6 CHEMISTRY MANUFACTURING AND CONTROLS (CMC) SERVICES
6.7 DATA MANAGEMENT SERVICES
6.8 OTHERS
7 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION
7.1 OVERVIEW
7.2 CLINICAL CHEMISTRY AND IMMUNOASSAYS
7.3 INFECTIOUS DISEASES
7.3.1 VIROLOGY
7.3.2 MICROBIOLOGY AND MYCOLOGY
7.3.3 BACTERIOLOGY
7.3.4 SEPSIS
7.3.5 HEPATITIS B
7.3.6 HEPATITIS C
7.3.7 MALARIA
7.3.8 TUBERCULOSIS
7.3.9 SYPHILIS
7.3.10 HUMAN PAPILLOMAVIRUS (HPV) INFECTION
7.3.11 OTHERS
7.4 HAEMATOLOGY
7.5 DRUG TESTING/PHARMACOGENOMICS
7.6 PRECISION MEDICINE
7.7 DIABETES
7.8 BLOOD TRANSFUSION
7.9 CARDIOLOGY
7.1 POINT OF CARE
7.10.1 WAIVED TEST
7.10.2 AT HOME TESTS
7.11 ONCOLOGY
7.12 NEUROLOGY
7.13 HIV/AIDS
7.14 GENETIC TESTING
7.15 OTHERS
8 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS
8.1 OVERVIEW
8.2 CLASS I
8.3 CLASS III
8.4 CLASS II
9 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE
9.1 OVERVIEW
9.2 CLOUD
9.3 ON-PREMISES
10 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE
10.1 OVERVIEW
10.2 LARGE ENTERPRISES
10.3 SMALL & MEDIUM ENTERPRISES (SMES)
11 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE
11.1 OVERVIEW
11.2 CLINICAL
11.3 PRECLINICAL
11.4 PMA (POST MARKET AUTHORIZATION)
12 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER
12.1 OVERVIEW
12.2 MEDICAL DEVICE COMPANIES
12.2.1 BY ORGANIZATION SIZE
12.2.1.1 LARGE ENTERPRISES
12.2.1.2 SMALL & MEDIUM ENTERPRISES (SMES)
12.2.2 BY SERVICE
12.2.2.1 REGULATORY WRITING & SUBMISSIONS
12.2.2.2 LEGAL REPRESENTATION
12.2.2.3 REGULATORY CONSULTING
12.2.2.4 REGULATORY REGISTRATION & CLINICAL TRIAL APPLICATIONS
12.2.2.5 CHEMISTRY MANUFACTURING AND CONTROLS (CMC) SERVICES
12.2.2.6 DATA MANAGEMENT SERVICES
12.2.2.7 OTHERS
12.3 PHARMACEUTICAL COMPANIES
12.3.1 BY ORGANIZATION SIZE
12.3.1.1 LARGE ENTERPRISES
12.3.1.2 SMALL & MEDIUM ENTERPRISES (SMES)
12.3.2 BY SERVICE
12.3.2.1 REGULATORY WRITING & SUBMISSIONS
12.3.2.2 LEGAL REPRESENTATION
12.3.2.3 REGULATORY CONSULTING
12.3.2.4 REGULATORY REGISTRATION & CLINICAL TRIAL APPLICATIONS
12.3.2.5 CHEMISTRY MANUFACTURING AND CONTROLS (CMC) SERVICES
12.3.2.6 DATA MANAGEMENT SERVICES
12.3.2.7 OTHERS
12.4 BIOTECHNOLOGY COMPANIES
12.4.1 BY ORGANIZATION SIZE
12.4.1.1 LARGE ENTERPRISES
12.4.1.2 SMALL & MEDIUM ENTERPRISES (SMES)
12.4.2 BY SERVICE
12.4.2.1 REGULATORY WRITING & SUBMISSIONS
12.4.2.2 LEGAL REPRESENTATION
12.4.2.3 REGULATORY CONSULTING
12.4.2.4 REGULATORY REGISTRATION & CLINICAL TRIAL APPLICATIONS
12.4.2.5 CHEMISTRY MANUFACTURING AND CONTROLS (CMC) SERVICES
12.4.2.6 DATA MANAGEMENT SERVICES
12.4.2.7 OTHERS
12.5 OTHERS
13 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION
13.1 ASIA-PACIFIC
13.1.1 CHINA
13.1.2 JAPAN
13.1.3 INDIA
13.1.4 SOUTH KOREA
13.1.5 AUSTRALIA
13.1.6 SINGAPORE
13.1.7 THAILAND
13.1.8 MALAYSIA
13.1.9 INDONESIA
13.1.10 PHILIPPINES
13.1.11 REST OF ASIA-PACIFIC
14 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: ASIA PACIFIC
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 ICON PLC
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENTS
16.2 MEDPACE
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 PAREXEL INTERNATIONAL CORPORATION
16.3.1 COMPANY SNAPSHOT
16.3.2 COMPANY SHARE ANALYSIS
16.3.3 PRODUCT PORTFOLIO
16.3.4 RECENT DEVELOPMENTS
16.4 LABCORP DRUG DEVELOPMENT
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT DEVELOPMENT
16.5 PPD INC. (A SUBSIDIARY OF THERMOFISHER SCIENTIFIC INC.)
16.5.1 COMPANY SNAPSHOT
16.5.2 COMPANY SHARE ANALYSIS
16.5.3 PRODUCT PORTFOLIO
16.5.4 RECENT DEVELOPMENTS
16.6 CHARLES RIVER LABORATORIES
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENTS
16.7 FREYR
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENTS
16.8 ASSENT COMPLIANCE INC.
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENT
16.9 ANDAMAN MEDICAL
16.9.1 COMPANY SNAPSHOT
16.9.2 PRODUCT PORTFOLIO
16.9.3 RECENT DEVELOPMENT
16.1 ASIA ACTUAL
16.10.1 COMPANY SNAPSHOT
16.10.2 SERVICE PORTFOLIO
16.10.3 RECENT DEVELOPMENTS
16.11 AXSOURCE
16.11.1 COMPANY SNAPSHOT
16.11.2 PRODUCT PORTFOLIO
16.11.3 RECENT DEVELOPMENTS
16.12 CRITERIUM, INC.
16.12.1 COMPANY SNAPSHOT
16.12.2 PRODUCT PORTFOLIO
16.12.3 RECENT DEVELOPMENT
16.13 DOR PHARMACEUTICAL SERVICES
16.13.1 COMPANY SNAPSHOT
16.13.2 SERVICE PORTFOLIO
16.13.3 RECENT DEVELOPMENTS
16.14 EMERGO BY UL
16.14.1 COMPANY SNAPSHOT
16.14.2 PRODUCT PORTFOLIO
16.14.3 RECENT DEVELOPMENTS
16.15 GENPACT
16.15.1 COMPANY SNAPSHOT
16.15.2 REVENUE ANALYSIS
16.15.3 PRODUCT PORTFOLIO
16.15.4 RECENT DEVELOPMENTS
16.16 GROUPE PRODUCTLIFE S.A.
16.16.1 COMPANY SNAPSHOT
16.16.2 PRODUCT PORTFOLIO
16.16.3 RECENT DEVELOPMENTS
16.17 LORENZ LIFE SCIENCES GROUP
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENTS
16.18 MAKROCARE
16.18.1 COMPANY SNAPSHOT
16.18.2 PRODUCT PORTFOLIO
16.18.3 RECENT DEVELOPMENTS
16.19 MARACA INTERNATIONAL BVBA
16.19.1 COMPANY SNAPSHOT
16.19.2 SERVICE PORTFOLIO
16.19.3 RECENT DEVELOPMENTS
16.2 MDICONSULTANTS, INC.
16.20.1 COMPANY SNAPSHOT
16.20.2 PRODUCT PORTFOLIO
16.20.3 RECENT DEVELOPMENTS
16.21 PBC BIOMED
16.21.1 COMPANY SNAPSHOT
16.21.2 PRODUCT PORTFOLIO
16.21.3 RECENT DEVELOPMENT
16.22 PROMEDICA INTERNATIONAL, A CALIFORNIA CORPORATION
16.22.1 COMPANY SNAPSHOT
16.22.2 PRODUCT PORTFOLIO
16.22.3 RECENT DEVELOPMENT
16.23 PROPHARMA GROUP
16.23.1 COMPANY SNAPSHOT
16.23.2 PRODUCT PORTFOLIO
16.23.3 RECENT DEVELOPMENTS
16.24 QSERVE
16.24.1 COMPANY SNAPSHOT
16.24.2 SERVICE PORTFOLIO
16.24.3 RECENT DEVELOPMENTS
16.25 REGULATORY COMPLIANCE ASSOCIATES INC.
16.25.1 COMPANY SNAPSHOT
16.25.2 PRODUCT PORTFOLIO
16.25.3 RECENT DEVELOPMENT
16.26 RMQ+
16.26.1 COMPANY SNAPSHOT
16.26.2 PRODUCT PORTFOLIO
16.26.3 RECENT DEVELOPMENT
16.27 SARACA SOLUTIONS PRIVATE LIMITED
16.27.1 COMPANY SNAPSHOT
16.27.2 PRODUCT PORTFOLIO
16.27.3 RECENT DEVELOPMENT
16.28 VCLS
16.28.1 COMPANY SNAPSHOT
16.28.2 PRODUCT PORTFOLIO
16.28.3 RECENT DEVELOPMENTS
16.29 WUXI APPTEC
16.29.1 COMPANY SNAPSHOT
16.29.2 REVENUE ANALYSIS
16.29.3 PRODUCT PORTFOLIO
16.29.4 RECENT DEVELOPMENTS
17 QUESTIONNAIRE
18 RELATED REPORTS
Lista de Tablas
TABLE 1 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 2 ASIA PACIFIC REGULATORY WRITING & SUBMISSIONS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 3 ASIA PACIFIC LEGAL REPRESENTATION IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 4 ASIA PACIFIC REGULATORY CONSULTING IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 5 ASIA PACIFIC REGULATORY REGISTRATION & CLINICAL TRIAL APPLICATIONS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 6 ASIA PACIFIC CHEMISTRY MANUFACTURING AND CONTROLS (CMC) SERVICES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 7 ASIA PACIFIC DATA MANAGEMENT SERVICES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY MATERIAL, 2020-2029 (USD THOUSAND)
TABLE 8 ASIA PACIFIC OTHERS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 9 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 10 ASIA PACIFIC CLINICAL CHEMISTRY AND IMMUNOASSAYS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 11 ASIA PACIFIC INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION,2020-2029 (THOUSAND)
TABLE 12 ASIA PACIFIC INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 13 ASIA PACIFIC HAEMATOLOGY IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 14 ASIA PACIFIC DRUG TESTING/PHARMACOGENOMICS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 15 ASIA PACIFIC PRECISION MEDICINE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 16 ASIA PACIFIC DIABETES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 17 ASIA PACIFIC BLOOD TRANSFUSION IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 18 ASIA PACIFIC CARDIOLOGY IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 19 ASIA PACIFIC POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 20 ASIA PACIFIC POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 21 ASIA PACIFIC ONCOLOGY IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 22 ASIA PACIFIC NEUROLOGY IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 23 ASIA PACIFIC HIV/AIDS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 24 ASIA PACIFIC GENETIC TESTING IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 25 ASIA PACIFIC OTHERS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 26 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 27 ASIA PACIFIC CLASS I IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 28 ASIA PACIFIC CLASS III IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 29 ASIA PACIFIC CLASS II IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 30 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 31 ASIA PACIFIC CLOUD IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 32 ASIA PACIFIC ON-PREMISES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 33 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 34 ASIA PACIFIC LARGE ENTERPRISES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 35 ASIA PACIFIC SMALL & MEDIUM ENTERPRISES (SMES) IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 36 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 37 ASIA PACIFIC CLINICAL IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 38 ASIA PACIFIC PRECLINICAL IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 39 ASIA PACIFIC PMA (POST MARKET AUTHORIZATION) IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 40 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 41 ASIA PACIFIC MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 42 ASIA PACIFIC MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 43 ASIA PACIFIC MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 44 ASIA PACIFIC PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 45 ASIA PACIFIC PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 46 ASIA PACIFIC PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 47 ASIA PACIFIC BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 48 ASIA PACIFIC BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 49 ASIA PACIFIC BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 50 ASIA PACIFIC OTHERS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 51 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY COUNTRY, 2020-2029 (USD THOUSAND)
TABLE 52 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 53 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 54 ASIA-PACIFIC INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 55 ASIA-PACIFIC POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 56 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 57 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 58 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 59 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 60 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 61 ASIA-PACIFIC MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 62 ASIA-PACIFIC MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 63 ASIA-PACIFIC PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 64 ASIA-PACIFIC PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 65 ASIA-PACIFIC BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 66 ASIA-PACIFIC BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 67 CHINA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 68 CHINA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 69 CHINA INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 70 CHINA POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 71 CHINA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 72 CHINA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 73 CHINA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 74 CHINA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 75 CHINA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 76 CHINA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 77 CHINA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 78 CHINA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 79 CHINA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 80 CHINA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 81 CHINA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 82 JAPAN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 83 JAPAN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 84 JAPAN INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 85 JAPAN POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 86 JAPAN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 87 JAPAN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 88 JAPAN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 89 JAPAN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 90 JAPAN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 91 JAPAN MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 92 JAPAN MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 93 JAPAN PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 94 JAPAN PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 95 JAPAN BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 96 JAPAN BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 97 INDIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 98 INDIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 99 INDIA INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 100 INDIA POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 101 INDIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 102 INDIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 103 INDIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 104 INDIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 105 INDIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 106 INDIA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 107 INDIA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 108 INDIA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 109 INDIA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 110 INDIA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 111 INDIA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 112 SOUTH KOREA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 113 SOUTH KOREA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 114 SOUTH KOREA INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 115 SOUTH KOREA POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 116 SOUTH KOREA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 117 SOUTH KOREA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 118 SOUTH KOREA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 119 SOUTH KOREA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 120 SOUTH KOREA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 121 SOUTH KOREA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 122 SOUTH KOREA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 123 SOUTH KOREA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 124 SOUTH KOREA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 125 SOUTH KOREA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 126 SOUTH KOREA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 127 AUSTRALIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 128 AUSTRALIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 129 AUSTRALIA INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 130 AUSTRALIA POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 131 AUSTRALIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 132 AUSTRALIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 133 AUSTRALIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 134 AUSTRALIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 135 AUSTRALIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 136 AUSTRALIA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 137 AUSTRALIA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 138 AUSTRALIA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 139 AUSTRALIA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 140 AUSTRALIA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 141 AUSTRALIA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 142 SINGAPORE IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 143 SINGAPORE IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 144 SINGAPORE INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 145 SINGAPORE POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 146 SINGAPORE IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 147 SINGAPORE IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 148 SINGAPORE IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 149 SINGAPORE IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 150 SINGAPORE IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 151 SINGAPORE MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 152 SINGAPORE MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 153 SINGAPORE PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 154 SINGAPORE PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 155 SINGAPORE BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 156 SINGAPORE BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 157 THAILAND IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 158 THAILAND IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 159 THAILAND INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 160 THAILAND POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 161 THAILAND IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 162 THAILAND IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 163 THAILAND IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 164 THAILAND IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 165 THAILAND IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 166 THAILAND MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 167 THAILAND MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 168 THAILAND PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 169 THAILAND PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 170 THAILAND BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 171 THAILAND BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 172 MALAYSIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 173 MALAYSIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 174 MALAYSIA INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 175 MALAYSIA POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 176 MALAYSIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 177 MALAYSIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 178 MALAYSIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 179 MALAYSIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 180 MALAYSIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 181 MALAYSIA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 182 MALAYSIA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 183 MALAYSIA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 184 MALAYSIA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 185 MALAYSIA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 186 MALAYSIA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 187 INDONESIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 188 INDONESIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 189 INDONESIA INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 190 INDONESIA POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 191 INDONESIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 192 INDONESIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 193 INDONESIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 194 INDONESIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 195 INDONESIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 196 INDONESIA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 197 INDONESIA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 198 INDONESIA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 199 INDONESIA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 200 INDONESIA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 201 INDONESIA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 202 PHILIPPINES IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 203 PHILIPPINES IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 204 PHILIPPINES INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 205 PHILIPPINES POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 206 PHILIPPINES IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 207 PHILIPPINES IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 208 PHILIPPINES IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 209 PHILIPPINES IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 210 PHILIPPINES IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 211 PHILIPPINES MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 212 PHILIPPINES MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 213 PHILIPPINES PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 214 PHILIPPINES PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 215 PHILIPPINES BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 216 PHILIPPINES BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 217 REST OF ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
Lista de figuras
FIGURE 1 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: SEGMENTATION
FIGURE 2 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: DATA TRIANGULATION
FIGURE 3 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: DROC ANALYSIS
FIGURE 4 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: ASIA PACIFIC VS REGIONAL MARKET ANALYSIS
FIGURE 5 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: DBMR MARKET POSITION GRID
FIGURE 8 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: VENDOR SHARE ANALYSIS
FIGURE 9 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: END USER COVERAGE GRID
FIGURE 10 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: SEGMENTATION
FIGURE 11 RISE IN THE PREVALENCE OF CHRONIC DISEASES ACROSS IS EXPECTED TO DRIVE ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 SERVICE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET IN 2022 & 2029
FIGURE 13 ASIA-PACIFIC IS EXPECTED TO DOMINATE AND IS THE FASTEST GROWING REGION IN THE ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES FOR ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET
FIGURE 15 MULTIPLE CHRONIC CONDITIONS AMONG PEOPLE AGED 65 AND ABOVE IN EUROPEAN REGION (2017)
FIGURE 16 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY SERVICE, 2021
FIGURE 17 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY INDICATION, 2021
FIGURE 18 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY CLASS, 2021
FIGURE 19 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY DEPLOYMENT MODE, 2021
FIGURE 20 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY ORGANIZATION SIZE, 2021
FIGURE 21 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY STAGE, 2021
FIGURE 22 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY END USER, 2021
FIGURE 23 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: SNAPSHOT (2021)
FIGURE 24 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY COUNTRY (2021)
FIGURE 25 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 26 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 27 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY SERVICE (2022-2029)
FIGURE 28 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: COMPANY SHARE 2021 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.