Europe Cancer Photodynamic Therapy Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
872.14 Million
USD
1,353.25 Million
2024
2032
USD
872.14 Million
USD
1,353.25 Million
2024
2032
| 2025 –2032 | |
| USD 872.14 Million | |
| USD 1,353.25 Million | |
|
|
|
|
Segmentación del mercado europeo de terapia fotodinámica contra el cáncer, por tipo de producto (fármacos fotosensibilizantes, dispositivos de terapia fotodinámica), por indicación oncológica (piel y oncología cutánea, cabeza y cuello, esófago, pulmón, vejiga, cuello uterino, próstata), por modalidad terapéutica (terapia única, terapia adyuvante, terapia paliativa, otras), por técnica de procedimiento (radioterapia externa, administración intracavitaria (endoscópica), administración intersticial (interna), otras), por estadio de la enfermedad (cáncer en estadio temprano, cáncer en estadio avanzado), por datos demográficos del paciente (geriátrico, adultos, pediátrico), por usuario final (hospitales, clínicas de dermatología y cáncer de piel, centros quirúrgicos ambulatorios, instituciones académicas y de investigación, otros), por canal de distribución (licitaciones directas, distribuidores externos, en línea, otros) - Tendencias del sector y previsiones hasta 2032
Tamaño del mercado europeo de terapia fotodinámica contra el cáncer
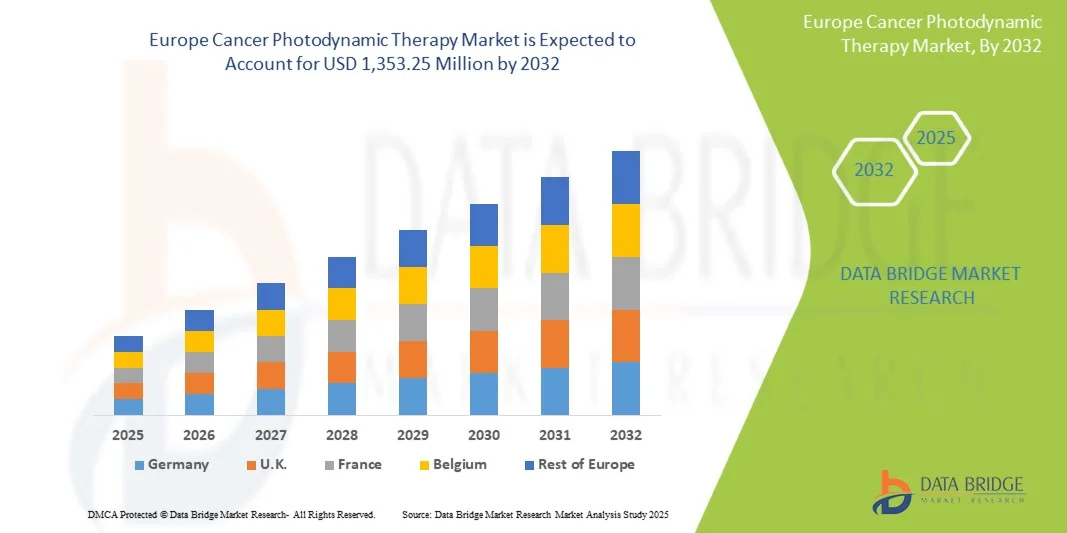
- El mercado europeo de terapia fotodinámica contra el cáncer se valoró en 872,14 millones de dólares en 2024 y se espera que alcance los 1.353,25 millones de dólares en 2032, con una tasa de crecimiento anual compuesta (TCAC) del 5,8% durante el período de previsión.
- El mercado está impulsado principalmente por la creciente prevalencia del cáncer, el aumento del gasto sanitario y la mayor concienciación sobre las opciones de tratamiento avanzadas. Las rápidas mejoras en la infraestructura sanitaria y la expansión de los centros especializados en el tratamiento del cáncer también contribuyen a este crecimiento.
- Este crecimiento se debe a factores como las iniciativas gubernamentales que promueven el diagnóstico precoz y las terapias innovadoras, la gran cantidad de pacientes y el aumento de las inversiones de empresas internacionales y locales en tecnologías de terapia fotodinámica.
Análisis del mercado europeo de terapia fotodinámica contra el cáncer
- El mercado de la terapia fotodinámica (TFD) contra el cáncer está experimentando un crecimiento constante, impulsado por la creciente prevalencia del cáncer, la mayor concienciación sobre los tratamientos no invasivos y los avances en fármacos fotosensibilizadores y tecnologías láser.
- Los mercados emergentes están experimentando una rápida adopción de la terapia fotodinámica (TFD), impulsada por iniciativas gubernamentales, el creciente gasto sanitario y el envejecimiento de la población. Sin embargo, los elevados costes del tratamiento y las limitaciones en el reembolso siguen siendo obstáculos importantes, mientras que las innovaciones en terapias combinadas y fotosensibilizadores específicos presentan importantes oportunidades de crecimiento.
- Se prevé que Alemania domine el mercado europeo de terapia fotodinámica contra el cáncer con la mayor cuota de ingresos, un 19,26%, en 2025. Este crecimiento se debe a su avanzada infraestructura sanitaria, la alta adopción de tratamientos innovadores, las fuertes inversiones en I+D, las políticas de reembolso favorables y el conocimiento de las terapias mínimamente invasivas.
- Se prevé que Alemania sea la región de mayor crecimiento en el mercado europeo de terapia fotodinámica contra el cáncer durante el período de pronóstico, con una tasa de crecimiento anual compuesto (TCAC) del 8,9%. Este crecimiento se debe al aumento de la prevalencia del cáncer, la expansión de la infraestructura sanitaria, la mayor concienciación sobre las terapias avanzadas y las iniciativas gubernamentales que promueven el diagnóstico precoz. Además, la creciente adopción de tecnologías innovadoras y el aumento de la renta disponible impulsan la demanda de terapia fotodinámica en la región.
- Se prevé que el segmento de fármacos fotosensibilizantes domine el mercado europeo de terapia fotodinámica contra el cáncer con una cuota de mercado del 75,15 % en 2025, impulsado por su papel fundamental en el tratamiento, su alta especificidad para atacar las células cancerosas, la creciente aprobación de nuevos fármacos, su mayor adopción en terapias combinadas y la continua I+D que impulsa una mayor eficacia y reduce los efectos secundarios.
Alcance del informe y segmentación del mercado europeo de terapia fotodinámica contra el cáncer
|
Atributos |
Información clave del mercado de la seda |
|
Segmentos cubiertos |
|
|
Países cubiertos |
Europa
|
|
Principales actores del mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de datos de valor añadido |
Además de los datos sobre escenarios de mercado como el valor de mercado, la tasa de crecimiento, la segmentación, la cobertura geográfica y los principales actores, los informes de mercado elaborados por Data Bridge Market Research también incluyen análisis de importación y exportación, descripción general de la capacidad de producción, análisis del consumo de producción, análisis de la tendencia de los precios, escenario de cambio climático, análisis de la cadena de suministro, análisis de la cadena de valor, descripción general de materias primas/consumibles, criterios de selección de proveedores, análisis PESTLE, análisis de Porter y marco regulatorio. |
Tendencias del mercado europeo de terapia fotodinámica contra el cáncer
“Integración con otras terapias contra el cáncer”
- La capacidad de la terapia fotodinámica (TFD) para producir la destrucción localizada de células tumorales al tiempo que estimula las respuestas inmunitarias la posiciona como un socio atractivo para el tratamiento multimodal del cáncer.
- Cada vez hay más evidencia que demuestra que la terapia fotodinámica (TFD) puede aumentar la liberación de antígenos tumorales, modular el microambiente tumoral y mejorar la infiltración o activación de células inmunitarias; mecanismos que pueden actuar en sinergia con los inhibidores de puntos de control inmunitario, las vacunas terapéuticas contra el cáncer, la quimioterapia o la radioterapia.
- La combinación de la terapia fotodinámica con terapias sistémicas puede convertir el control local en respuestas sistémicas duraderas, permitir reducciones de dosis de agentes tóxicos y ampliar las indicaciones (por ejemplo, enfermedad irresecable o metastásica).
- A medida que se multiplican las investigaciones clínicas y traslacionales, la integración con otras modalidades representa una vía de gran valor para ampliar la relevancia clínica y la comercialización de la PDT.
Dinámica del mercado europeo de terapia fotodinámica contra el cáncer
Conductor
“Aumento de la prevalencia del cáncer”
- La creciente prevalencia del cáncer a nivel mundial es uno de los principales factores que impulsan la demanda de terapias como la terapia fotodinámica (TFD).
- A medida que las poblaciones crecen y envejecen, y a medida que mejoran las herramientas de diagnóstico, se detectan más casos de cáncer cada año.
- Las mayores tasas de factores de riesgo como el consumo de tabaco, la obesidad, el sedentarismo, la contaminación atmosférica y las infecciones en los países de ingresos bajos y medios también están contribuyendo al aumento de la incidencia.
- Con un número creciente de pacientes que requieren modalidades de tratamiento localizado eficaces, menos invasivas y rentables, la terapia fotodinámica (TFD) se vuelve más atractiva.
- Esta creciente carga de cáncer ejerce presión sobre los sistemas de salud, creando una necesidad urgente de terapias que puedan mejorar los resultados, reducir los efectos secundarios y aplicarse de forma más generalizada.
Restricción/Desafío
“Profundidad limitada de penetración de la luz”
- Una limitación importante que dificulta la adopción generalizada y la eficacia de la terapia fotodinámica es la penetración restringida de la luz activadora en los tejidos humanos.
- Dado que los fotosensibilizadores deben activarse mediante luz de longitudes de onda específicas, la absorción y dispersión de la luz por el tejido reducen la profundidad a la que puede llegar la iluminación.
- Los fotosensibilizadores de luz visible suelen ser eficaces solo para tumores superficiales o de fácil acceso; los tumores más profundos o de mayor tamaño siguen presentando dificultades.
- Esta limitación conlleva una destrucción incompleta del tumor, requiere la administración de luz invasiva (por ejemplo, sondas de fibra, endoscopia), aumenta la complejidad del procedimiento y puede provocar malos resultados o recurrencia.
- Hasta que se logren avances que superen este obstáculo, la terapia fotodinámica (TFD) sigue estando limitada en cuanto al tipo de cánceres que puede tratar de forma no invasiva y eficaz.
Alcance del mercado europeo de terapia fotodinámica contra el cáncer
El mercado está segmentado en función del tipo de producto, la indicación del cáncer, la modalidad de terapia, la técnica del procedimiento, el estadio de la enfermedad, la demografía del paciente, el usuario final y el canal de distribución.
- Por tipo de producto
Según el tipo de producto, el mercado europeo de terapia fotodinámica contra el cáncer se segmenta en fármacos fotosensibilizadores y dispositivos de terapia fotodinámica. Se prevé que en 2025, los fármacos fotosensibilizadores dominen el mercado con una cuota del 75,15 %, debido a su papel fundamental en la eficacia del tratamiento, su amplia aplicabilidad en diversos tipos de cáncer y sus versátiles formulaciones (intravenosa, tópica, oral, intravesical e intraperitoneal). Entre los factores clave que impulsan este dominio se encuentran la creciente prevalencia del cáncer, la mayor adopción de terapias mínimamente invasivas, las continuas innovaciones farmacológicas y las aprobaciones regulatorias, que en conjunto convierten a los fotosensibilizadores en la principal fuente de ingresos por encima de los dispositivos de terapia fotodinámica.
Los fármacos fotosensibilizantes constituyen el segmento de mayor crecimiento en el mercado europeo de terapia fotodinámica contra el cáncer, con una tasa de crecimiento anual compuesto (TCAC) del 5,9%, debido a la creciente adopción de tratamientos oncológicos dirigidos y mínimamente invasivos. La mayor concienciación sobre la eficacia de la terapia fotodinámica, sus menores efectos secundarios en comparación con las terapias convencionales y el desarrollo de fotosensibilizadores de última generación con mayor selectividad tumoral y penetración tisular más profunda impulsan la demanda. Asimismo, la investigación clínica y las aprobaciones de nuevos agentes fotosensibilizantes contribuyen a la expansión del mercado.
- Por indicación de cáncer
Según la indicación oncológica, el mercado europeo de terapia fotodinámica (TFD) para el cáncer se segmenta en oncología cutánea, cabeza y cuello, esófago, pulmón, vejiga, cuello uterino y próstata. Se prevé que en 2025, el segmento de oncología cutánea domine el mercado con una cuota del 52,21%, debido a la alta prevalencia del cáncer de piel, la creciente concienciación sobre la detección precoz y la eficacia de la TFD para lograr resultados estéticos superiores. Este segmento se beneficia de la amplia adopción de fármacos fotosensibilizantes y dispositivos de TFD, especialmente en pacientes geriátricos y adultos, que representan el mayor grupo demográfico. Además, la creciente demanda de terapias mínimamente invasivas y dirigidas para la queratosis actínica, el carcinoma basocelular y el carcinoma espinocelular, junto con políticas de reembolso favorables en regiones clave, refuerza aún más su liderazgo en el mercado frente a otras indicaciones oncológicas.
El segmento de Oncología Cutánea es el de mayor crecimiento, con una tasa de crecimiento anual compuesto (TCAC) del 6,3 % en el mercado europeo de terapia fotodinámica para el cáncer. Esto se debe a la creciente prevalencia del cáncer de piel, la mayor concienciación sobre el diagnóstico precoz y la preferencia por tratamientos mínimamente invasivos con menos efectos secundarios. La terapia fotodinámica ofrece una acción dirigida, una recuperación rápida y mejores resultados estéticos, lo que la convierte en una opción muy favorable para la oncología dermatológica. Además, los avances tecnológicos en fotosensibilizadores y sistemas de administración de luz están impulsando su adopción en este segmento.
- Por modalidad de terapia
Según la modalidad terapéutica, el mercado europeo de terapia fotodinámica (TFD) para el cáncer se segmenta en terapia independiente, terapia adyuvante, terapia paliativa y otras. Se prevé que en 2025, el segmento de terapia independiente domine el mercado con una cuota del 41,20 %, debido a su eficacia como tratamiento primario para cánceres localizados, incluidos los de piel, esófago y pulmón. Los factores clave que impulsan este dominio incluyen su alta eficacia, mínima invasividad, excelentes resultados estéticos y una creciente preferencia clínica por las terapias dirigidas. A nivel regional, Norteamérica y Europa lideran la adopción de la TFD independiente gracias a su avanzada infraestructura sanitaria, sus marcos de reembolso establecidos y la alta concienciación de los pacientes, mientras que los mercados emergentes de Asia-Pacífico experimentan una creciente adopción impulsada por el aumento de la prevalencia del cáncer, la expansión de las redes hospitalarias y el mayor acceso a tratamientos oncológicos modernos. Estas dinámicas regionales, junto con una mayor educación y concienciación sobre los beneficios de la TFD, refuerzan el dominio de la terapia independiente a nivel mundial.
La terapia fotodinámica oncológica en monoterapia es el segmento de mayor crecimiento, con una tasa de crecimiento anual compuesto (TCAC) del 6,2%, debido a su simplicidad, rentabilidad y menores efectos secundarios en comparación con las terapias combinadas. Permite el tratamiento dirigido de tumores sin necesidad de fármacos o intervenciones adicionales, lo que mejora la adherencia al tratamiento. Su creciente adopción en entornos ambulatorios, la mayor concienciación sobre los tratamientos mínimamente invasivos y los avances en fotosensibilizadores y sistemas de administración de luz impulsan aún más el rápido crecimiento de este segmento.
- Mediante técnica de procedimiento
Según la técnica empleada, el mercado europeo de terapia fotodinámica contra el cáncer se segmenta en haz externo, administración intracavitaria (endoscópica), administración intersticial (interna) y otras. Se prevé que en 2025, el segmento de haz externo domine el mercado con una cuota del 68,97 %, debido a su carácter no invasivo, facilidad de uso y eficacia en tumores superficiales. Su sólida adopción en Norteamérica y Europa, respaldada por una infraestructura sanitaria avanzada y políticas de reembolso favorables, junto con la creciente demanda en Asia-Pacífico derivada del aumento de la prevalencia y la concienciación sobre el cáncer, impulsa su liderazgo en el mercado.
El segmento de administración intracavitaria (endoscópica) es el de mayor crecimiento, con una tasa de crecimiento anual compuesto (TCAC) del 6,3 % en el mercado europeo de terapia fotodinámica contra el cáncer, ya que permite la administración mínimamente invasiva y dirigida de luz y fotosensibilizador a tumores de órganos huecos, reduce la exposición sistémica y los efectos secundarios, permite tratamientos repetibles, mejora el acceso al tumor en cánceres de esófago, bronquios y vejiga, y acorta el tiempo de recuperación.
- Por etapa de la enfermedad
Según la etapa de la enfermedad, el mercado europeo de terapia fotodinámica contra el cáncer se divide en cáncer en etapa temprana y cáncer en etapa avanzada. Se prevé que en 2025, el segmento de cáncer en etapa temprana domine el mercado con una cuota del 81,51 %, debido a la eficacia de la terapia fotodinámica para tratar tumores localizados, minimizar el daño al tejido sano y ofrecer mejores resultados estéticos. Este segmento se beneficia de una alta concienciación por parte de los pacientes, la preferencia por tratamientos mínimamente invasivos y su amplia adopción en Norteamérica y Europa, mientras que el aumento de las tasas de diagnóstico de cáncer y la expansión de la infraestructura oncológica en Asia-Pacífico refuerzan aún más su liderazgo en el mercado.
El cáncer en estadio temprano es el segmento de mayor crecimiento, con una tasa de crecimiento anual compuesto (TCAC) del 5,8 % en el mercado europeo de terapia fotodinámica oncológica. Esto se debe a la creciente adopción de tratamientos mínimamente invasivos, la mayor concienciación sobre la detección precoz y la mejora de los resultados para los pacientes con la terapia fotodinámica. Los cánceres en estadio temprano responden mejor a las terapias dirigidas, lo que se traduce en una mayor eficacia y menos efectos secundarios. Además, las iniciativas gubernamentales de apoyo y los avances en fotosensibilizadores y sistemas de administración de luz están impulsando una mayor penetración en el mercado de este segmento.
- Por datos demográficos del paciente
Según las características demográficas de los pacientes, el mercado europeo de terapia fotodinámica oncológica se segmenta en geriátrico, adultos y pediátrico. Se prevé que en 2025 el segmento geriátrico domine el mercado con una cuota del 67,12 %, debido a la mayor prevalencia de cáncer en adultos mayores, la mayor susceptibilidad a los cánceres de piel y cutáneos, y la preferencia por tratamientos mínimamente invasivos y dirigidos. La fuerte adopción de esta terapia en Norteamérica y Europa se ve respaldada por una infraestructura sanitaria avanzada y una mayor concienciación, junto con el creciente envejecimiento de la población.
El segmento geriátrico es el de mayor crecimiento, con una tasa de crecimiento anual compuesto (TCAC) del 6,0 % en el mercado europeo de terapia fotodinámica oncológica, debido a la mayor prevalencia de cáncer entre los adultos mayores. El envejecimiento debilita el sistema inmunitario y aumenta la susceptibilidad a diversos tipos de cáncer, lo que impulsa la demanda de tratamientos eficaces y mínimamente invasivos como la TFD. Además, la TFD ofrece menos efectos secundarios y una recuperación más rápida, lo que la hace idónea para pacientes de edad avanzada que podrían no tolerar terapias agresivas, impulsando así el crecimiento del mercado en este grupo demográfico.
- Por usuario final
Según el usuario final, el mercado europeo de terapia fotodinámica oncológica se segmenta en hospitales, clínicas de dermatología y cáncer de piel, centros quirúrgicos ambulatorios, instituciones académicas y de investigación, y otros. Se prevé que en 2025, el segmento de hospitales domine el mercado con una cuota del 41,33 %, gracias a su infraestructura integral, la disponibilidad de departamentos de oncología especializados y su capacidad para ofrecer tratamientos de terapia fotodinámica integrados. Tanto los hospitales públicos como los privados, en particular los de nivel 1 y 2 en Norteamérica y Europa, lideran la adopción de esta terapia debido a sus sistemas sanitarios avanzados y al apoyo de los sistemas de reembolso. El crecimiento de las redes hospitalarias y la expansión de los servicios oncológicos en Asia-Pacífico refuerzan aún más el dominio de los hospitales como principales usuarios finales de la terapia fotodinámica oncológica a nivel mundial.
El sector hospitalario es el de mayor crecimiento en el mercado europeo de terapia fotodinámica oncológica, con una tasa de crecimiento anual compuesto (TCAC) del 6,5%. Esto se debe a la creciente adopción de tratamientos oncológicos avanzados, el mayor flujo de pacientes y la disponibilidad de departamentos de oncología especializados. Los hospitales ofrecen servicios integrales de TFD, que incluyen diagnóstico, tratamiento y cuidados posteriores, lo que los convierte en la opción preferida frente a las clínicas independientes. Además, la creciente concienciación, las iniciativas gubernamentales y la cobertura de los seguros impulsan aún más la adopción de la TFD en el ámbito hospitalario.
- Por canal de distribución
Según el canal de distribución, el mercado europeo de terapia fotodinámica contra el cáncer se segmenta en licitación directa, distribuidores externos, venta en línea y otros. Se prevé que en 2025, el segmento de licitación directa domine el mercado con una cuota del 50,79 %, debido a las compras al por mayor realizadas por hospitales, programas de salud pública y grandes centros oncológicos, lo que garantiza la rentabilidad y el suministro fiable de fotosensibilizadores y dispositivos de terapia fotodinámica. La fuerte adopción en Norteamérica y Europa, respaldada por sistemas estructurados de compras hospitalarias y licitaciones de salud pública, junto con una creciente demanda institucional, también contribuye a este crecimiento.
El segmento de licitación directa es el de mayor crecimiento, con una tasa de crecimiento anual compuesto (TCAC) del 6,0 % en el mercado europeo de terapia fotodinámica oncológica, debido al aumento de las adquisiciones de dispositivos PDT avanzados por parte de gobiernos y hospitales mediante contratos directos. Este enfoque garantiza la rentabilidad, una adquisición más rápida y un suministro fiable para programas de tratamiento oncológico a gran escala. Además, el aumento del gasto público en sanidad, las iniciativas gubernamentales para la atención oncológica y la preferencia por las compras centralizadas impulsan la adopción de la licitación directa frente a los distribuidores o los canales en línea.
Análisis regional del mercado europeo de terapia fotodinámica contra el cáncer
- Se prevé que Alemania domine el mercado europeo de terapia fotodinámica contra el cáncer con la mayor cuota de ingresos, un 19,26%, en 2025. Este crecimiento se debe a su avanzada infraestructura sanitaria, la alta adopción de tratamientos innovadores, las fuertes inversiones en I+D, las políticas de reembolso favorables y el conocimiento de las terapias mínimamente invasivas.
- Se prevé que Alemania sea la región de mayor crecimiento en el mercado europeo de terapia fotodinámica contra el cáncer durante el período de pronóstico, con una tasa de crecimiento anual compuesto (TCAC) del 8,9%. Este crecimiento se debe al aumento de la prevalencia del cáncer, la expansión de la infraestructura sanitaria, la mayor concienciación sobre las terapias avanzadas y las iniciativas gubernamentales que promueven el diagnóstico precoz. Además, la creciente adopción de tecnologías innovadoras y el aumento de la renta disponible impulsan la demanda de terapia fotodinámica en la región.
- Además, la presencia de los principales actores del mercado y de marcos regulatorios favorables acelera el crecimiento del mercado y las tasas de adopción en la región.
Perspectivas del mercado de la terapia fotodinámica contra el cáncer en el Reino Unido y Europa
El mercado de la terapia fotodinámica (TFD) contra el cáncer en el Reino Unido y Europa desempeña un papel fundamental en la industria europea de plásticos médicos, impulsado por su sólida infraestructura sanitaria, sus avanzadas capacidades de investigación y sus centros oncológicos de gran prestigio. La alta adopción de terapias innovadoras, el firme apoyo gubernamental al tratamiento del cáncer y los ensayos clínicos en curso impulsan el crecimiento del mercado. Además, la colaboración entre empresas farmacéuticas e instituciones de investigación en el Reino Unido acelera el desarrollo y la comercialización de la TFD, convirtiéndolo en un actor clave del mercado europeo.
Perspectivas del mercado de la terapia fotodinámica contra el cáncer en Alemania y Europa
Se prevé que el mercado de terapia fotodinámica (TFD) para el cáncer en Alemania y Europa experimente un crecimiento sostenido, impulsado por una infraestructura sanitaria avanzada, la alta adopción de tratamientos oncológicos innovadores y un sólido apoyo gubernamental a la investigación oncológica. La creciente concienciación sobre las terapias mínimamente invasivas, la mayor incidencia de cánceres como el de piel y pulmón, y las políticas de reembolso para terapias novedosas fomentan aún más su adopción. Además, la presencia de fabricantes clave de dispositivos de TFD y las colaboraciones en investigación aceleran la penetración en el mercado y el crecimiento sostenido.
Los principales líderes del mercado que operan en el mercado son:
- Novartis Pharma AG (Suiza)
- Galderma SA (Suiza)
- Bausch Health Companies Inc. (Canadá)
- Fotocurado ASA (Noruega)
- ADVANZ PHARMA Corp. (Reino Unido)
- Sun Pharmaceutical Industries Ltd. (India)
- Biofrontera AG (Alemania)
- LUMIBIRD SA (Francia)
- LUZITIN SA (Portugal)
- Lumeda Inc. (Suecia)
- ImPact Biotech (Israel)
- biolitec Holding GmbH & Co KG (Alemania)
- Corporación Modulight (Finlandia)
- (Canadá)
Últimos avances en el mercado europeo de la terapia fotodinámica contra el cáncer
- En febrero de 2023, la colaboración entre Galderma y German Medical Engineering (GME) representa un avance estratégico en el mercado de la dermatología y la terapia fotodinámica (TFD). Al combinar Metvix de Galderma, un fotosensibilizador líder para lesiones precancerosas y cánceres de piel no melanoma, con el dispositivo MultiLite de GME, esta alianza fortalece la oferta de tratamientos integrados de Galderma y amplía su capacidad para administrar tanto la TFD convencional con luz roja (TFD-C) como la TFD con luz diurna artificial (TFD-LDA), más cómoda para el paciente.
- En 2025, McKesson completó la adquisición de Core Ventures (Community Oncology Revitalization Enterprise Ventures), adquiriendo aproximadamente el 70% de la participación mayoritaria por unos 2.490 millones de dólares estadounidenses, para fortalecer su atención oncológica comunitaria a través de Florida Cancer Specialists & Research Institute.
- En 2025, Biofrontera AG transfirió todos los activos estadounidenses relacionados con Ameluz y RhodoLED a Biofrontera Inc., recibiendo una participación accionaria del 10% y regalías del 12-15% sobre las ventas de Ameluz en Estados Unidos.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE CANCER PHOTODYNAMIC THERAPY MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 MARKET END USER COVERAGE GRID
2.1 SECONDARY SOURCES
2.11 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER’S FIVE FORCES
4.2 PESTEL ANALYSIS
4.3 PRODUCTION CONSUMPTION ANALYSIS
4.3.1 INTRODUCTION
4.3.2 PRODUCTION SIDE ANALYSIS
4.3.2.1 PHOTOSENSITIZER MANUFACTURING
4.3.2.2 DEVICE MANUFACTURING
4.3.2.3 RESEARCH AND INNOVATION
4.3.3 CONSUMPTION SIDE ANALYSIS
4.3.3.1 CLINICAL APPLICATION
4.3.3.2 TREATMENT VOLUMES AND TRENDS
4.3.3.3 DOSAGE AND PROTOCOLS
4.3.4 PRODUCTION–CONSUMPTION DYNAMICS
4.3.4.1 SUPPLY CONSTRAINTS
4.3.4.2 REGIONAL OVERVIEW
4.3.4.3 FUTURE OUTLOOK
4.3.5 CONCLUSION
4.4 COST ANALYSIS BREAKDOWN
4.4.1 INTRODUCTION
4.4.2 DIRECT MEDICAL COSTS
4.4.2.1 COST OF PHOTOSENSITIZERS
4.4.2.2 LIGHT DELIVERY SYSTEMS
4.4.2.3 HEALTHCARE FACILITY CHARGES
4.4.3 INDIRECT COSTS
4.4.3.1 PATIENT-RELATED EXPENSES
4.4.3.2 POST-TREATMENT MONITORING
4.4.4 COMPARATIVE COST-EFFECTIVENESS
4.4.5 REIMBURSEMENT AND INSURANCE IMPACT
4.4.6 REGIONAL COST VARIATIONS
4.4.7 FUTURE COST TRENDS AND REDUCTION STRATEGIES
4.4.7.1 TECHNOLOGICAL ADVANCEMENTS
4.4.7.2 HEALTHCARE EFFICIENCY INITIATIVES
4.4.8 CONCLUSION
4.5 TECHNOLOGICAL ADVANCEMENTS
4.5.1 INTRODUCTION
4.5.2 NEXT-GENERATION PHOTOSENSITIZERS
4.5.3 ADVANCEMENTS IN LIGHT DELIVERY SYSTEMS
4.5.4 NANOTECHNOLOGY-ENABLED DELIVERY
4.5.5 COMBINATION THERAPIES AND IMMUNOMODULATION
4.5.6 DIGITAL INTEGRATION AND TREATMENT PLANNING
4.5.7 RECENT TRENDS AND OUTLOOK
4.5.8 CONCLUSION
4.6 VALUE CHAIN ANALYSIS
4.6.1 INTRODUCTION
4.6.2 RESEARCH & DEVELOPMENT
4.6.2.1 DISCOVERY OF PHOTOSENSITIZERS
4.6.2.2 DEVELOPMENT OF LIGHT DELIVERY SYSTEMS
4.6.2.3 CLINICAL TRIALS AND REGULATORY APPROVALS
4.6.3 MANUFACTURING
4.6.3.1 PRODUCTION OF PHOTOSENSITIZERS
4.6.3.2 FABRICATION OF LIGHT DELIVERY DEVICES
4.6.4 DISTRIBUTION & LOGISTICS
4.6.4.1 SUPPLY CHAIN MANAGEMENT
4.6.4.2 INTERNATIONAL TRADE AND MARKET ACCESS
4.6.5 CLINICAL APPLICATION
4.6.5.1 INTEGRATION INTO TREATMENT PROTOCOLS
4.6.5.2 TRAINING AND EDUCATION
4.6.6 POST-TREATMENT MONITORING & SUPPORT
4.6.6.1 FOLLOW-UP CARE
4.6.6.2 PATIENT SUPPORT SERVICES
4.6.7 TECHNOLOGICAL ADVANCEMENTS INFLUENCING THE PDT VALUE CHAIN
4.6.7.1 NANOTECHNOLOGY IN PDT
4.6.7.2 ARTIFICIAL INTELLIGENCE AND IMAGING
4.6.7.3 PERSONALIZED MEDICINE
4.6.8 CONCLUSION
4.7 VENDOR SELECTION CRITERIA
4.7.1 INTRODUCTION
4.7.2 CORE SELECTION CRITERIA
4.7.2.1 REGULATORY COMPLIANCE
4.7.2.2 CLINICAL EVIDENCE AND RESEARCH SUPPORT
4.7.2.3 TECHNICAL PERFORMANCE AND DEVICE COMPATIBILITY
4.7.2.4 QUALITY MANAGEMENT AND MANUFACTURING STANDARDS
4.7.2.5 SERVICE, TRAINING, AND AFTER-SALES SUPPORT
4.7.2.6 SUPPLY CHAIN RELIABILITY
4.7.3 RECENT TRENDS IN VENDOR SELECTION
4.7.4 RISK FACTORS AND VULNERABILITIES
4.7.5 KEY PERFORMANCE INDICATORS
4.7.6 STRATEGIC RECOMMENDATIONS
4.7.7 CONCLUSION
4.8 PATENT ANALYSIS
4.8.1 PATENT QUALITY AND STRENGTH
4.8.2 PATENT FAMILIES
4.8.3 LICENSING AND COLLABORATIONS
4.8.4 REGIONAL PATENT LANDSCAPE
4.8.5 IP STRATEGY AND MANAGEMENT
4.9 SUPPLY CHAIN ANALYSIS
4.9.1 OVERVIEW
4.9.2 LOGISTIC COST SCENARIO
4.9.3 IMPORTANCE OF LOGISTICS SERVICE PROVIDERS
4.9.4 CONCLUSION
4.1 INDUSTRY ECOSYSTEM ANALYSIS
4.10.1 INTRODUCTION
4.10.2 ECOSYSTEM ARCHITECTURE — KEY ACTORS AND ROLES
4.10.2.1 CORE TECHNOLOGY PROVIDERS
4.10.2.2 ENABLING INSTITUTIONS
4.10.3 VALUE CHAIN AND FUNCTIONAL FLOWS
4.10.3.1 RESEARCH AND DISCOVERY
4.10.3.2 CLINICAL DEVELOPMENT AND REGULATORY VALIDATION
4.10.3.3 MANUFACTURING AND QUALITY ASSURANCE
4.10.3.4 DISTRIBUTION, PROCUREMENT AND CLINICAL ADOPTION
4.10.4 MARKET ENABLERS AND INFRASTRUCTURE
4.10.4.1 SCIENTIFIC AND REGULATORY ENABLERS
4.10.4.2 REIMBURSEMENT AND HEALTH-ECONOMICS INFRASTRUCTURE
4.10.4.3 MANUFACTURING AND SUPPLY-CHAIN CAPACITY
4.10.5 INTERDEPENDENCIES AND STRATEGIC PARTNERSHIPS
4.10.5.1 ACADEMIA-INDUSTRY TECHNOLOGY TRANSFER
4.10.5.2 VERTICAL INTEGRATION AND CONTRACT MANUFACTURING
4.10.5.3 CLINICAL NETWORKS AND KOL ECOSYSTEMS
4.10.6 RISKS, CONSTRAINTS AND SYSTEMIC VULNERABILITIES
4.10.6.1 REGULATORY COMPLEXITY FOR COMBINED PRODUCTS
4.10.6.2 SUPPLY-CHAIN CONCENTRATION AND MATERIAL RISK
4.10.6.3 EVIDENCE AND REIMBURSEMENT UNCERTAINTY
4.10.6.4 CLINICAL OPERATIONAL BARRIERS
4.10.7 STRATEGIC IMPLICATIONS AND RECOMMENDATIONS
4.10.8 OUTLOOK — EVOLUTION OF THE ECOSYSTEM
4.10.9 CONCLUSION
4.11 INNOVATION TRACKER AND STRATEGIC ANALYSIS
4.11.1 INTRODUCTION
4.11.2 RECENT TECHNOLOGICAL INNOVATIONS
4.11.2.1 ADVANCED PHOTOSENSITIZERS
4.11.2.2 OXYGEN-SELF-SUFFICIENT PLATFORMS
4.11.2.3 ALTERNATIVE ACTIVATION MODALITIES
4.11.2.4 SMART NANOPLATFORMS
4.11.2.5 NOVEL CHEMICAL STRUCTURES
4.11.3 STRATEGIC INNOVATIONS IN DELIVERY SYSTEMS
4.11.3.1 LIGHT DELIVERY DEVICES
4.11.3.2 COMBINATION THERAPIES
4.11.3.3 IMAGING INTEGRATION
4.11.4 KEY CHALLENGES
4.11.5 STRATEGIC THEMES
4.11.6 STRATEGIC IMPLICATIONS FOR MARKET PLAYERS
4.11.7 RECOMMENDATIONS
4.11.8 OUTLOOK AND STRATEGIC RISKS
4.11.9 CONCLUSION
4.12 PRICING ANALYSIS
4.12.1 INTRODUCTION
4.12.2 COMPONENTS OF THE TOTAL TREATMENT PRICE
4.12.2.1 PHOTOSENSITIZING AGENT (DRUG) COSTS
4.12.2.2 DEVICE CAPITAL AND MAINTENANCE COST
4.12.2.3 CONSUMABLES AND PROCEDURAL OVERHEAD
4.12.2.4 INDIRECT AND DOWNSTREAM COSTS
4.12.3 PRICING MODELS AND APPROACHES
4.12.3.1 COST-PLUS AND MARKUP MODELS
4.12.3.2 VALUE-BASED AND OUTCOMES-LINKED PRICING
4.12.3.3 BUNDLED PAYMENTS AND PROCEDURAL TARIFFS
4.12.3.4 SUBSCRIPTION AND MANAGED-SERVICE MODELS FOR DEVICES
4.12.4 REIMBURSEMENT LANDSCAPE
4.12.4.1 UNITED STATES: MEDICARE AND COMMERCIAL PAYERS
4.12.4.2 EUROPE AND OTHER HIGH-INCOME MARKETS
4.12.4.3 EMERGING MARKETS AND OUT-OF-POCKET DYNAMICS
4.12.5 REGIONAL PRICE DIFFERENTIALS AND DRIVERS
4.12.5.1 MANUFACTURING FOOTPRINT AND SUPPLY-CHAIN EFFECTS
4.12.5.2 REGULATORY BURDEN AND MARKET ACCESS TIMELINES
4.12.5.3 CLINICAL PRACTICE PATTERNS AND REIMBURSEMENT POLICY
4.12.6 PRICE SENSITIVITY, ACCESS, AND EQUITY
4.12.6.1 PRICE ELASTICITY IN HOSPITAL PROCUREMENT
4.12.6.2 PATIENT ACCESS AND SOCIOECONOMIC BARRIERS
4.12.7 COMPETITIVE & STRATEGIC PRICING IMPLICATIONS
4.12.7.1 DIFFERENTIATION-BASED PREMIUM PRICING
4.12.7.2 PENETRATION PRICING AND VOLUME STRATEGIES
4.12.7.3 MANAGED ENTRY AGREEMENTS AND OUTCOMES GUARANTEES
4.12.8 RECOMMENDATIONS FOR STAKEHOLDERS
4.12.8.1 FOR MANUFACTURERS
4.12.8.2 FOR PROVIDERS AND HOSPITAL SYSTEMS
4.12.8.3 FOR PAYERS AND POLICYMAKERS
4.12.9 RISKS, UNCERTAINTIES, AND FUTURE PRICE PRESSURES
4.12.10 CONCLUSION
5 TARIFFS & IMPACT ON THE MARKET
5.1 INTRODUCTION
5.2 TARIFF LANDSCAPE RELEVANT TO PDT PRODUCTS
5.2.1 CATEGORIES OF TRADE EXPOSURE
5.2.2 RECENT AND EMERGING TARIFF MEASURES OF CONSEQUENCE
5.3 DIRECT COST IMPACTS
5.3.1 INCREASED LANDED COSTS AND MARGIN COMPRESSION
5.3.2 PRICE VOLATILITY AND PROCUREMENT BUDGETING
5.4 SUPPLY-CHAIN & MANUFACTURING IMPLICATIONS
5.4.1 SUPPLIER DIVERSIFICATION AND RESHORING INCENTIVES
5.4.2 SOURCING OF HIGH-VALUE NANOMATERIALS AND COMPONENTS
5.4.3 REGULATORY AND QUALIFICATION COSTS FOR NEW SUPPLIERS
5.5 CLINICAL ACCESS, PRICING & REIMBURSEMENT EFFECTS
5.5.1 ACCESS RISK FOR PATIENTS AND PROVIDERS
5.5.2 REIMBURSEMENT PRESSURE AND HEALTH-ECONOMIC ASSESSMENTS
5.6 R&D, INNOVATION & COMPETITIVE IMPLICATIONS
5.6.1 DISRUPTION OF RESEARCH SUPPLIES AND COLLABORATION FLOWS
5.6.2 STRATEGIC REPOSITIONING AND COMPETITIVE ADVANTAGE
5.7 POLICY, COMPLIANCE & REGULATORY CONSIDERATIONS
5.7.1 USE OF WTO AND PREFERENTIAL TRADE RULES
5.7.2 TARIFF MITIGATION TOOLS AND ADVOCACY
5.8 RECOMMENDATIONS FOR STAKEHOLDERS
5.9 CONCLUSION
6 REGULATION COVERAGE
6.1 PRODUCT CODES
6.1.1 CERTIFIED STANDARDS
6.1.2 SAFETY STANDARDS
6.1.3 MATERIAL HANDLING & STORAGE
6.1.4 TRANSPORT & PRECAUTIONS
6.1.5 HAZARD IDENTIFICATION
6.1.6 CONCLUSION
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 INCREASING PREVALENCE OF CANCER
7.1.2 GROWING PREFERENCE FOR MINIMALLY INVASIVE THERAPIES
7.1.3 TECHNOLOGICAL ADVANCEMENTS IN PHOTOSENSITIZERS AND DEVICES
7.1.4 EXPANDING RESEARCH AND CLINICAL DEVELOPMENT PIPELINE
7.2 RESTRAINTS
7.2.1 LIMITED DEPTH OF LIGHT PENETRATION
7.2.2 HIGH COST OF TREATMENT
7.3 OPPORTUNITIES
7.3.1 INTEGRATION WITH OTHER CANCER THERAPIES
7.3.2 DEVELOPMENT OF NOVEL PHOTOSENSITIZERS
7.3.3 M&A AND PARTNERSHIPS WITH ONCOLOGY DEVICE/LASER FIRMS AND PHARMA
7.4 CHALLENGES
7.4.1 TUMOR HYPOXIA AS A BIOLOGICAL BARRIER TO PHOTODYNAMIC THERAPY EFFICACY
7.4.2 COMPETITION FROM ALTERNATIVE TREATMENTS
8 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE
8.1 OVERVIEW
8.2 PHOTOSENSITIZER DRUGS
8.3 PHOTODYNAMIC THERAPY DEVICES
9 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION
9.1 OVERVIEW
9.2 SKIN & CUTANEOUS ONCOLOGY
9.3 HEAD & NECK
9.4 ESOPHAGAL
9.5 LUNG
9.6 BLADDER
9.7 CERVICAL
9.8 PROSTATE
10 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY
10.1 OVERVIEW
10.2 STANDALONE THERAPY
10.3 ADJUNCTIVE THERAPY
10.4 PALLIATION THERAPY
10.5 OTHERS
11 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE
11.1 OVERVIEW
11.2 EXTERNAL BEAM
11.3 INTRACAVITARY (ENDOSCOPIC) DELIVERY
11.4 INTERSTITIAL (INTERNAL) DELIVERY
11.5 OTHERS
12 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE
12.1 OVERVIEW
12.2 EARLY-STAGE CANCER
12.3 LATE-STAGE CANCER
13 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS
13.1 OVERVIEW
13.2 GERIATRIC
13.3 ADULTS
13.4 PEDIATRIC
14 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER
14.1 OVERVIEW
14.2 HOSPITALS
14.3 DERMATOLOGY & SKIN-CANCER CLINICS
14.4 AMBULATORY SURGICAL CENTERS (ASCS)
14.5 ACADEMIC & RESEARCH INSTITUTES
14.6 OTHERS
15 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL
15.1 OVERVIEW
15.2 DIRECT TENDER
15.3 THIRD PARTY DISTRIBUTORS
15.4 ONLINE
15.5 OTHERS
16 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION
16.1 EUROPE
16.1.1 GERMANY
16.1.2 U.K.
16.1.3 FRANCE
16.1.4 ITALY
16.1.5 SPAIN
16.1.6 RUSSIA
16.1.7 TURKEY
16.1.8 SWITZERLAND
16.1.9 BELGIUM
16.1.10 NETHERLANDS
16.1.11 SWEDEN
16.1.12 DENMARK
16.1.13 NORWAY
16.1.14 FINLAND
16.1.15 REST OF EUROPE
17 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET: COMPANY LANDSCAPE
17.1 COMPANY SHARE ANALYSIS: GLOBAL
18 SWOT ANALYSIS
19 COMPANY PROFILE
19.1 NOVERTIS AG
19.1.1 COMPANY SNAPSHOT
19.1.2 REVENUE ANALYSIS
19.1.3 COMPANY SHARE ANALYSIS
19.1.4 PRODUCT PORTFOLIO
19.1.5 RECENT DEVELOPMENT
19.2 GALDERMA S. A.
19.2.1 COMPANY SNAPSHOT
19.2.2 REVENUE ANALYSIS
19.2.3 COMPANY SHARE ANALYSIS
19.2.4 PRODUCT PORTFOLIO
19.2.5 RECENT DEVELOPMENTS
19.3 PHOTOCURE
19.3.1 COMPANY SNAPSHOT
19.3.2 REVENUE ANALYSIS
19.3.3 COMPANY SHARE ANALYSIS
19.3.4 PRODUCT PORTFOLIO
19.3.5 RECENT DEVELOPMENT
19.4 ADVANZ PHARMA CORP.
19.4.1 COMPANY SNAPSHOT
19.4.2 COMPANY SHARE ANALYSIS
19.4.3 PRODUCT PORTFOLIO
19.4.4 RECENT DEVELOPMENT
19.5 AMERISOURCE BERGEN CORPORATION
19.5.1 COMPANY SNAPSHOT
19.5.2 REVENUE ANALYSIS
19.5.3 COMPANY SHARE ANALYSIS
19.5.4 PRODUCT PORTFOLIO
19.5.5 RECENT DEVELOPMENT
19.6 BIOFRONTERA AG
19.6.1 COMPANY SNAPSHOT
19.6.2 REVENUE ANALYSIS
19.6.3 PRODUCT PORTFOLIO
19.6.4 RECENT DEVELOPMENTS
19.7 BIOLITEC HOLDING GMBH & CO KG
19.7.1 COMPANY SNAPSHOT
19.7.2 PRODUCT PORTFOLIO
19.7.3 RECENT DEVELOPMENT
19.8 CARDINAL HEALTH
19.8.1 COMPANY SNAPSHOT
19.8.2 REVENUE ANALYSIS
19.8.3 PRODUCT PORTFOLIO
19.8.4 RECENT DEVELOPMENT
19.9 HEMERION THERAPEUTICS
19.9.1 COMPANY SNAPSHOT
19.9.2 PRODUCT PORTFOLIO
19.9.3 RECENT DEVELOPMENTS
19.1 IMPACT BIOTECH
19.10.1 COMPANY SNAPSHOT
19.10.2 PRODUCT PORTFOLIO
19.10.3 RECENT DEVELOPMENTS
19.11 INOVA
19.11.1 COMPANY SNAPSHOT
19.11.2 PRODUCT PORTFOLIO
19.11.3 RECENT DEVELOPMENT
19.12 LUMIBIRD
19.12.1 COMPANY SNAPSHOT
19.12.2 REVENUE ANALYSIS
19.12.3 PRODUCT PORTFOLIO
19.12.4 RECENT DEVELOPMENT
19.13 LUZITIN
19.13.1 COMPANY SNAPSHOT
19.13.2 PRODUCT PORTFOLIO
19.13.3 RECENT DEVELOPMENT
19.14 MCKESSON
19.14.1 COMPANY SNAPSHOT
19.14.2 REVENUE ANALYSIS
19.14.3 PRODUCT PORTFOLIO
19.14.4 RECENT DEVELOPMENTS
19.15 MODULIGHT CORPORATION
19.15.1 COMPANY SNAPSHOT
19.15.2 REVENUE ANALYSIS
19.15.3 PRODUCT PORTFOLIO
19.15.4 RECENT DEVELOPMENT
19.16 ONCOLUX INC (FORMERLY LUMEDA INC.)
19.16.1 COMPANY SNAPSHOT
19.16.2 PRODUCT PORTFOLIO
19.16.3 RECENT DEVELOPMENT
19.17 SUN PHARMACEUTICAL INDUSTRIES LTD
19.17.1 COMPANY SNAPSHOT
19.17.2 REVENUE ANALYSIS
19.17.3 PRODUCT PORTFOLIO
19.17.4 RECENT DEVELOPMENT
19.18 THERALASE TECHNOLOGIES INC.
19.18.1 COMPANY SNAPSHOT
19.18.2 REVENUE ANALYSIS
19.18.3 PRODUCT PORTFOLIO
19.18.4 RECENT DEVELOPMENT
20 QUESTIONNAIRE
21 RELATED REPORTS
Lista de Tablas
TABLE 1 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 2 EUROPE PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2019-2028 (USD THOUSAND)
TABLE 3 EUROPE PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 4 EUROPE PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 5 EUROPE PHOTODYNAMIC THERAPY DEVICES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 6 EUROPE PHOTODYNAMIC THERAPY DEVICES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 7 EUROPE LIGHT DELIVERY SYSTEMS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 8 EUROPE ACCESSORIES & CONSUMABLES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 9 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION, 2018-2032 (USD THOUSAND)
TABLE 10 EUROPE SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 11 EUROPE SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 12 EUROPE SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 13 EUROPE HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 14 EUROPE HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 15 EUROPE HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 16 EUROPE ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 17 EUROPE ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 18 EUROPE ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 19 EUROPE LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 20 EUROPE LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 21 EUROPE LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 22 EUROPE BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 23 EUROPE BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 24 EUROPE BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 25 EUROPE CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 26 EUROPE CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 27 EUROPE CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 28 EUROPE PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 29 EUROPE PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 30 EUROPE PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 31 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY, 2018-2032 (USD THOUSAND)
TABLE 32 EUROPE STANDALONE THERAPY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 33 EUROPE ADJUNCTIVE THERAPY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 34 EUROPE PALLIATION THERAPY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 35 EUROPE OTHERS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 36 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE, 2018-2032 (USD THOUSAND)
TABLE 37 EUROPE EXTERNAL BEAM IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 38 EUROPE INTRACAVITARY (ENDOSCOPIC) DELIVERY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 39 EUROPE INTERSTITIAL (INTERNAL) DELIVERY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 40 EUROPE OTHERS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 41 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE, 2018-2032 (USD THOUSAND)
TABLE 42 EUROPE EARLY-STAGE CANCER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 43 EUROPE LATE-STAGE CANCER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 44 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 45 EUROPE GERIATRIC IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 46 EUROPE ADULTS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 47 EUROPE PEDIATRIC IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 48 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 49 EUROPE HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 50 EUROPE HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 51 EUROPE HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 52 EUROPE DERMATOLOGY & SKIN-CANCER CLINICS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 53 EUROPE AMBULATORY SURGICAL CENTERS (ASCS) IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 54 EUROPE ACADEMIC & RESEARCH INSTITUTES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 55 EUROPE OTHERS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 56 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 57 EUROPE DIRECT TENDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 58 EUROPE THIRD PARTY DISTRIBUTORS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 59 EUROPE ONLINE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 60 EUROPE OTHERS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 61 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 62 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 63 EUROPE PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 64 EUROPE PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 65 EUROPE PHOTODYNAMIC THERAPY DEVICES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 66 EUROPE LIGHT DELIVERY SYSTEMS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 67 EUROPE ACCESSORIES & CONSUMABLES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 68 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION, 2018-2032 (USD THOUSAND)
TABLE 69 EUROPE SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 70 EUROPE SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 71 EUROPE HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 72 EUROPE HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 73 EUROPE ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 74 EUROPE ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 75 EUROPE LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 76 EUROPE LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 77 EUROPE BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 78 EUROPE BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 79 EUROPE CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 80 EUROPE CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 81 EUROPE PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 82 EUROPE PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 83 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY, 2018-2032 (USD THOUSAND)
TABLE 84 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE, 2018-2032 (USD THOUSAND)
TABLE 85 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE, 2018-2032 (USD THOUSAND)
TABLE 86 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 87 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 88 EUROPE HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 89 EUROPE HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 90 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 91 GERMANY CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 92 GERMANY PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 93 GERMANY PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 94 GERMANY PHOTODYNAMIC THERAPY DEVICES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 95 GERMANY LIGHT DELIVERY SYSTEMS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 96 GERMANY ACCESSORIES & CONSUMABLES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 97 GERMANY CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION, 2018-2032 (USD THOUSAND)
TABLE 98 GERMANY SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 99 GERMANY SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 100 GERMANY HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 101 GERMANY HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 102 GERMANY ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 103 GERMANY ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 104 GERMANY LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 105 GERMANY LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 106 GERMANY BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 107 GERMANY BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 108 GERMANY CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 109 GERMANY CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 110 GERMANY PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 111 GERMANY PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 112 GERMANY CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY, 2018-2032 (USD THOUSAND)
TABLE 113 GERMANY CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE, 2018-2032 (USD THOUSAND)
TABLE 114 GERMANY CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE, 2018-2032 (USD THOUSAND)
TABLE 115 GERMANY CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 116 GERMANY CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 117 GERMANY HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 118 GERMANY HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 119 GERMANY CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 120 U.K. CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 121 U.K. PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 122 U.K. PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 123 U.K. PHOTODYNAMIC THERAPY DEVICES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 124 U.K. LIGHT DELIVERY SYSTEMS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 125 U.K. ACCESSORIES & CONSUMABLES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 126 U.K. CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION, 2018-2032 (USD THOUSAND)
TABLE 127 U.K. SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 128 U.K. SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 129 U.K. HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 130 U.K. HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 131 U.K. ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 132 U.K. ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 133 U.K. LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 134 U.K. LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 135 U.K. BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 136 U.K. BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 137 U.K. CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 138 U.K. CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 139 U.K. PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 140 U.K. PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 141 U.K. CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY, 2018-2032 (USD THOUSAND)
TABLE 142 U.K. CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE, 2018-2032 (USD THOUSAND)
TABLE 143 U.K. CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE, 2018-2032 (USD THOUSAND)
TABLE 144 U.K. CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 145 U.K. CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 146 U.K. HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 147 U.K. HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 148 U.K. CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 149 FRANCE CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 150 FRANCE PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 151 FRANCE PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 152 FRANCE PHOTODYNAMIC THERAPY DEVICES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 153 FRANCE LIGHT DELIVERY SYSTEMS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 154 FRANCE ACCESSORIES & CONSUMABLES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 155 FRANCE CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION, 2018-2032 (USD THOUSAND)
TABLE 156 FRANCE SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 157 FRANCE SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 158 FRANCE HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 159 FRANCE HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 160 FRANCE ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 161 FRANCE ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 162 FRANCE LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 163 FRANCE LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 164 FRANCE BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 165 FRANCE BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 166 FRANCE CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 167 FRANCE CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 168 FRANCE PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 169 FRANCE PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 170 FRANCE CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY, 2018-2032 (USD THOUSAND)
TABLE 171 FRANCE CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE, 2018-2032 (USD THOUSAND)
TABLE 172 FRANCE CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE, 2018-2032 (USD THOUSAND)
TABLE 173 FRANCE CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 174 FRANCE CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 175 FRANCE HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 176 FRANCE HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 177 FRANCE CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 178 ITALY CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 179 ITALY PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 180 ITALY PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 181 ITALY PHOTODYNAMIC THERAPY DEVICES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 182 ITALY LIGHT DELIVERY SYSTEMS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 183 ITALY ACCESSORIES & CONSUMABLES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 184 ITALY CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION, 2018-2032 (USD THOUSAND)
TABLE 185 ITALY SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 186 ITALY SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 187 ITALY HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 188 ITALY HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 189 ITALY ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 190 ITALY ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 191 ITALY LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 192 ITALY LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 193 ITALY BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 194 ITALY BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 195 ITALY CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 196 ITALY CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 197 ITALY PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 198 ITALY PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 199 ITALY CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY, 2018-2032 (USD THOUSAND)
TABLE 200 ITALY CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE, 2018-2032 (USD THOUSAND)
TABLE 201 ITALY CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE, 2018-2032 (USD THOUSAND)
TABLE 202 ITALY CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 203 ITALY CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 204 ITALY HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 205 ITALY HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 206 ITALY CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 207 SPAIN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 208 SPAIN PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 209 SPAIN PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 210 SPAIN PHOTODYNAMIC THERAPY DEVICES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 211 SPAIN LIGHT DELIVERY SYSTEMS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 212 SPAIN ACCESSORIES & CONSUMABLES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 213 SPAIN CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION, 2018-2032 (USD THOUSAND)
TABLE 214 SPAIN SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 215 SPAIN SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 216 SPAIN HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 217 SPAIN HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 218 SPAIN ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 219 SPAIN ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 220 SPAIN LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 221 SPAIN LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 222 SPAIN BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 223 SPAIN BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 224 SPAIN CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 225 SPAIN CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 226 SPAIN PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 227 SPAIN PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 228 SPAIN CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY, 2018-2032 (USD THOUSAND)
TABLE 229 SPAIN CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE, 2018-2032 (USD THOUSAND)
TABLE 230 SPAIN CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE, 2018-2032 (USD THOUSAND)
TABLE 231 SPAIN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 232 SPAIN CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 233 SPAIN HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 234 SPAIN HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 235 SPAIN CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 236 RUSSIA CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 237 RUSSIA PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 238 RUSSIA PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 239 RUSSIA PHOTODYNAMIC THERAPY DEVICES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 240 RUSSIA LIGHT DELIVERY SYSTEMS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 241 RUSSIA ACCESSORIES & CONSUMABLES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 242 RUSSIA CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION, 2018-2032 (USD THOUSAND)
TABLE 243 RUSSIA SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 244 RUSSIA SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 245 RUSSIA HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 246 RUSSIA HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 247 RUSSIA ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 248 RUSSIA ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 249 RUSSIA LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 250 RUSSIA LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 251 RUSSIA BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 252 RUSSIA BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 253 RUSSIA CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 254 RUSSIA CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 255 RUSSIA PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 256 RUSSIA PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 257 RUSSIA CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY, 2018-2032 (USD THOUSAND)
TABLE 258 RUSSIA CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE, 2018-2032 (USD THOUSAND)
TABLE 259 RUSSIA CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE, 2018-2032 (USD THOUSAND)
TABLE 260 RUSSIA CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 261 RUSSIA CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 262 RUSSIA HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 263 RUSSIA HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 264 RUSSIA CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 265 TURKEY CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 266 TURKEY PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 267 TURKEY PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 268 TURKEY PHOTODYNAMIC THERAPY DEVICES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 269 TURKEY LIGHT DELIVERY SYSTEMS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 270 TURKEY ACCESSORIES & CONSUMABLES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 271 TURKEY CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION, 2018-2032 (USD THOUSAND)
TABLE 272 TURKEY SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 273 TURKEY SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 274 TURKEY HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 275 TURKEY HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 276 TURKEY ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 277 TURKEY ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 278 TURKEY LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 279 TURKEY LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 280 TURKEY BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 281 TURKEY BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 282 TURKEY CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 283 TURKEY CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 284 TURKEY PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 285 TURKEY PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 286 TURKEY CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY, 2018-2032 (USD THOUSAND)
TABLE 287 TURKEY CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE, 2018-2032 (USD THOUSAND)
TABLE 288 TURKEY CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE, 2018-2032 (USD THOUSAND)
TABLE 289 TURKEY CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 290 TURKEY CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 291 TURKEY HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 292 TURKEY HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 293 TURKEY CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 294 SWITZERLAND CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 295 SWITZERLAND PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 296 SWITZERLAND PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 297 SWITZERLAND PHOTODYNAMIC THERAPY DEVICES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 298 SWITZERLAND LIGHT DELIVERY SYSTEMS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 299 SWITZERLAND ACCESSORIES & CONSUMABLES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 300 SWITZERLAND CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION, 2018-2032 (USD THOUSAND)
TABLE 301 SWITZERLAND SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 302 SWITZERLAND SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 303 SWITZERLAND HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 304 SWITZERLAND HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 305 SWITZERLAND ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 306 SWITZERLAND ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 307 SWITZERLAND LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 308 SWITZERLAND LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 309 SWITZERLAND BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 310 SWITZERLAND BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 311 SWITZERLAND CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 312 SWITZERLAND CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 313 SWITZERLAND PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 314 SWITZERLAND PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 315 SWITZERLAND CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY, 2018-2032 (USD THOUSAND)
TABLE 316 SWITZERLAND CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE, 2018-2032 (USD THOUSAND)
TABLE 317 SWITZERLAND CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE, 2018-2032 (USD THOUSAND)
TABLE 318 SWITZERLAND CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 319 SWITZERLAND CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 320 SWITZERLAND HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 321 SWITZERLAND HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 322 SWITZERLAND CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 323 BELGIUM CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 324 BELGIUM PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 325 BELGIUM PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 326 BELGIUM PHOTODYNAMIC THERAPY DEVICES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 327 BELGIUM LIGHT DELIVERY SYSTEMS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 328 BELGIUM ACCESSORIES & CONSUMABLES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 329 BELGIUM CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION, 2018-2032 (USD THOUSAND)
TABLE 330 BELGIUM SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 331 BELGIUM SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 332 BELGIUM HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 333 BELGIUM HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 334 BELGIUM ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 335 BELGIUM ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 336 BELGIUM LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 337 BELGIUM LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 338 BELGIUM BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 339 BELGIUM BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 340 BELGIUM CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 341 BELGIUM CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 342 BELGIUM PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 343 BELGIUM PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 344 BELGIUM CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY, 2018-2032 (USD THOUSAND)
TABLE 345 BELGIUM CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE, 2018-2032 (USD THOUSAND)
TABLE 346 BELGIUM CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE, 2018-2032 (USD THOUSAND)
TABLE 347 BELGIUM CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 348 BELGIUM CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 349 BELGIUM HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 350 BELGIUM HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 351 BELGIUM CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 352 NETHERLANDS CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 353 NETHERLANDS PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 354 NETHERLANDS PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 355 NETHERLANDS PHOTODYNAMIC THERAPY DEVICES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 356 NETHERLANDS LIGHT DELIVERY SYSTEMS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 357 NETHERLANDS ACCESSORIES & CONSUMABLES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 358 NETHERLANDS CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION, 2018-2032 (USD THOUSAND)
TABLE 359 NETHERLANDS SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 360 NETHERLANDS SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 361 NETHERLANDS HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 362 NETHERLANDS HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 363 NETHERLANDS ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 364 NETHERLANDS ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 365 NETHERLANDS LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 366 NETHERLANDS LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 367 NETHERLANDS BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 368 NETHERLANDS BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 369 NETHERLANDS CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 370 NETHERLANDS CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 371 NETHERLANDS PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 372 NETHERLANDS PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 373 NETHERLANDS CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY, 2018-2032 (USD THOUSAND)
TABLE 374 NETHERLANDS CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE, 2018-2032 (USD THOUSAND)
TABLE 375 NETHERLANDS CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE, 2018-2032 (USD THOUSAND)
TABLE 376 NETHERLANDS CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 377 NETHERLANDS CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 378 NETHERLANDS HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 379 NETHERLANDS HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 380 NETHERLANDS CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 381 SWEDEN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 382 SWEDEN PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 383 SWEDEN PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 384 SWEDEN PHOTODYNAMIC THERAPY DEVICES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 385 SWEDEN LIGHT DELIVERY SYSTEMS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 386 SWEDEN ACCESSORIES & CONSUMABLES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 387 SWEDEN CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION, 2018-2032 (USD THOUSAND)
TABLE 388 SWEDEN SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 389 SWEDEN SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 390 SWEDEN HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 391 SWEDEN HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 392 SWEDEN ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 393 SWEDEN ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 394 SWEDEN LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 395 SWEDEN LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 396 SWEDEN BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 397 SWEDEN BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 398 SWEDEN CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 399 SWEDEN CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 400 SWEDEN PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 401 SWEDEN PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 402 SWEDEN CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY, 2018-2032 (USD THOUSAND)
TABLE 403 SWEDEN CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE, 2018-2032 (USD THOUSAND)
TABLE 404 SWEDEN CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE, 2018-2032 (USD THOUSAND)
TABLE 405 SWEDEN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 406 SWEDEN CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 407 SWEDEN HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 408 SWEDEN HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 409 SWEDEN CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 410 DENMARK CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 411 DENMARK PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 412 DENMARK PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 413 DENMARK PHOTODYNAMIC THERAPY DEVICES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 414 DENMARK LIGHT DELIVERY SYSTEMS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 415 DENMARK ACCESSORIES & CONSUMABLES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 416 DENMARK CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION, 2018-2032 (USD THOUSAND)
TABLE 417 DENMARK SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 418 DENMARK SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 419 DENMARK HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 420 DENMARK HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 421 DENMARK ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 422 DENMARK ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 423 DENMARK LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 424 DENMARK LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 425 DENMARK BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 426 DENMARK BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 427 DENMARK CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 428 DENMARK CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 429 DENMARK PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 430 DENMARK PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 431 DENMARK CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY, 2018-2032 (USD THOUSAND)
TABLE 432 DENMARK CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE, 2018-2032 (USD THOUSAND)
TABLE 433 DENMARK CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE, 2018-2032 (USD THOUSAND)
TABLE 434 DENMARK CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 435 DENMARK CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 436 DENMARK HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 437 DENMARK HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 438 DENMARK CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 439 NORWAY CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 440 NORWAY PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 441 NORWAY PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 442 NORWAY PHOTODYNAMIC THERAPY DEVICES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 443 NORWAY LIGHT DELIVERY SYSTEMS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 444 NORWAY ACCESSORIES & CONSUMABLES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 445 NORWAY CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION, 2018-2032 (USD THOUSAND)
TABLE 446 NORWAY SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 447 NORWAY SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 448 NORWAY HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 449 NORWAY HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 450 NORWAY ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 451 NORWAY ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 452 NORWAY LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 453 NORWAY LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 454 NORWAY BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 455 NORWAY BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 456 NORWAY CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 457 NORWAY CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 458 NORWAY PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 459 NORWAY PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 460 NORWAY CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY, 2018-2032 (USD THOUSAND)
TABLE 461 NORWAY CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE, 2018-2032 (USD THOUSAND)
TABLE 462 NORWAY CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE, 2018-2032 (USD THOUSAND)
TABLE 463 NORWAY CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 464 NORWAY CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 465 NORWAY HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 466 NORWAY HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 467 NORWAY CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 468 FINLAND CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 469 FINLAND PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 470 FINLAND PHOTOSENSITIZER DRUGS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 471 FINLAND PHOTODYNAMIC THERAPY DEVICES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 472 FINLAND LIGHT DELIVERY SYSTEMS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 473 FINLAND ACCESSORIES & CONSUMABLES IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 474 FINLAND CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION, 2018-2032 (USD THOUSAND)
TABLE 475 FINLAND SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 476 FINLAND SKIN & CUTANEOUS ONCOLOGY IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 477 FINLAND HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 478 FINLAND HEAD & NECK IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 479 FINLAND ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 480 FINLAND ESOPHAGAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 481 FINLAND LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 482 FINLAND LUNG IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 483 FINLAND BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 484 FINLAND BLADDER IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 485 FINLAND CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 486 FINLAND CERVICAL IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 487 FINLAND PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 488 FINLAND PROSTATE IN CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 489 FINLAND CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY, 2018-2032 (USD THOUSAND)
TABLE 490 FINLAND CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE, 2018-2032 (USD THOUSAND)
TABLE 491 FINLAND CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE, 2018-2032 (USD THOUSAND)
TABLE 492 FINLAND CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032 (USD THOUSAND)
TABLE 493 FINLAND CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 494 FINLAND HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 495 FINLAND HOSPITALS IN CANCER PHOTODYNAMIC THERAPY MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 496 FINLAND CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 497 REST OF EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
Lista de figuras
FIGURE 1 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET: SEGMENTATION
FIGURE 2 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET: DROC ANALYSIS
FIGURE 4 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET: EUROPE VS REGIONAL ANALYSIS
FIGURE 5 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET: MARKET END USER COVERAGE GRID
FIGURE 9 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET: SEGMENTATION
FIGURE 10 EXECUTIVE SUMMARY
FIGURE 11 STRATEGIC DECISIONS
FIGURE 12 TWO SEGMENTS COMPRISE THE EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE (2024)
FIGURE 13 INCREASING PREVALENCE OF CANCER ACROSS GLOBE EXPECTED TO DRIVE THE EUROPE CANCER PHOTODYNAMIC THERAPY MARKET IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 14 PHOTOSENSITIZER DRUGS SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE CANCER PHOTODYNAMIC THERAPY MARKET IN 2025 & 2032
FIGURE 15 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET-MARKET OVERVIEW
FIGURE 16 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2024
FIGURE 17 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2024
FIGURE 18 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032
FIGURE 19 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PRODUCT TYPE, 2018-2032
FIGURE 20 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION, 2024
FIGURE 21 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION, 2024
FIGURE 22 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION, 2018-2032
FIGURE 23 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY CANCER INDICATION, 2018-2032
FIGURE 24 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY, 2024
FIGURE 25 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY, 2024
FIGURE 26 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY, 2018-2032
FIGURE 27 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY THERAPY MODALITY, 2018-2032
FIGURE 28 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE, 2024
FIGURE 29 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE, 2024
FIGURE 30 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE, 2018-2032
FIGURE 31 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PROCEDURE TECHNIQUE, 2018-2032
FIGURE 32 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE, 2024
FIGURE 33 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE, 2024
FIGURE 34 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE, 2018-2032
FIGURE 35 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY DISEASE STAGE, 2018-2032
FIGURE 36 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2024
FIGURE 37 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032
FIGURE 38 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032
FIGURE 39 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY PATIENT DEMOGRAPHICS, 2018-2032
FIGURE 40 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER, 2024
FIGURE 41 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER, 2024
FIGURE 42 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER, 2018-2032
FIGURE 43 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY END USER, 2018-2032
FIGURE 44 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2024
FIGURE 45 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2024
FIGURE 46 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2018-2032
FIGURE 47 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET, BY DISTRIBUTION CHANNEL, 2018-2032
FIGURE 48 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET: SNAPSHOT (2024)
FIGURE 49 EUROPE CANCER PHOTODYNAMIC THERAPY MARKET: COMPANY SHARE 2024 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.