Europe Graft Versus Host Disease Gvhd Treatment Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
583.49 Million
USD
823.47 Million
2024
2032
USD
583.49 Million
USD
823.47 Million
2024
2032
| 2025 –2032 | |
| USD 583.49 Million | |
| USD 823.47 Million | |
|
|
|
|
Mercado europeo de tratamiento de la enfermedad de injerto contra huésped (EICH), por tratamiento (medicamentos y terapia), tipo (EICH crónica, EICH aguda y profiláctico), género (femenino y masculino), edad (adultos y pediátricos), vía de administración (oral, intravenosa, tópica y otras), usuario final (hospitales, centros de trasplante, institutos y centros especializados), canal de distribución (licitación directa, venta minorista y otros): tendencias del sector y pronóstico hasta 2032
Tamaño del mercado europeo de tratamiento de la enfermedad de injerto contra huésped (EICH)
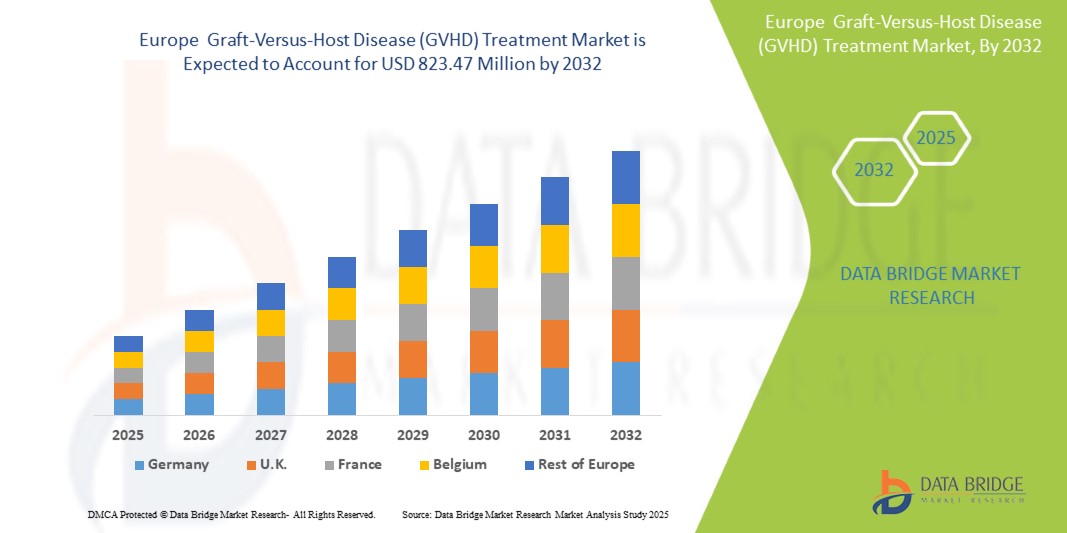
- El mercado europeo de tratamiento de la enfermedad de injerto contra huésped (EICH) se valoró en 583,49 millones de dólares en 2024 y se espera que alcance los 823,47 millones de dólares en 2032.
- Durante el período de pronóstico de 2025 a 2032, es probable que el mercado crezca a una CAGR del 4,5 %, impulsado principalmente por la creciente prevalencia de la obesidad.
- Este crecimiento se debe a factores como las innovaciones en medicamentos contra la enfermedad de injerto contra huésped (EICH) y el crecimiento de las cirugías bariátricas y metabólicas. Además, la integración de soluciones de salud digital en el tratamiento de la enfermedad de injerto contra huésped (EICH).
Análisis del mercado europeo de tratamiento de la enfermedad de injerto contra huésped (EICH)
- El creciente número de trasplantes de células madre y los avances en los enfoques de tratamiento son algunos de los principales factores que impulsan el crecimiento del mercado.
- Además, el desarrollo de nuevas terapias impulsa aún más el crecimiento del mercado. Sin embargo, la principal limitación que lo afecta es el alto costo del tratamiento.
- Por otro lado, se espera que una mayor concienciación y educación sobre la EICH entre profesionales de la salud, pacientes y cuidadores genere una oportunidad para el crecimiento del mercado. Sin embargo, se prevé que el riesgo de infecciones y otras complicaciones represente un desafío para dicho crecimiento.
- América del Norte sigue siendo una región dominante en el mercado europeo de tratamiento de la enfermedad de injerto contra huésped (EICH), respaldada por una infraestructura de atención médica bien establecida, altas tasas de prevalencia e investigación continua en soluciones de control de peso.
- Con un énfasis creciente en el manejo de la enfermedad de injerto contra huésped (EICH) y la atención médica preventiva, el mercado europeo está siendo testigo de importantes inversiones en nuevas terapias, tecnologías médicas y soluciones centradas en el paciente, lo que impulsa la expansión general de la industria.
Alcance del informe y segmentación del mercado de tratamiento de la enfermedad de injerto contra huésped (EICH)
|
Atributos |
Perspectivas clave del mercado europeo de tratamiento de la enfermedad de injerto contra huésped (EICH) |
|
Segmentos cubiertos |
|
|
Países cubiertos |
Europa
|
|
Actores clave del mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de información de datos de valor añadido |
Además de los conocimientos sobre escenarios de mercado, como valor de mercado, tasa de crecimiento, segmentación, cobertura geográfica y actores principales, los informes de mercado seleccionados por Data Bridge Market Research también incluyen análisis de importación y exportación, descripción general de la capacidad de producción, análisis del consumo de producción, análisis de tendencias de precios, escenario de cambio climático, análisis de la cadena de suministro, análisis de la cadena de valor, descripción general de materias primas/consumibles, criterios de selección de proveedores, análisis PESTLE, análisis de Porter y marco regulatorio. |
Tendencias del mercado de tratamiento de la enfermedad de injerto contra huésped (EICH) en Europa.
Aumento de la incidencia de trasplantes de células madre hematopoyéticas (TCMH)
- Esta tendencia se debe principalmente a la creciente prevalencia de cánceres hematológicos, como la leucemia, el linfoma y el mieloma, que a menudo requieren el TPH como opción de tratamiento. Además, otros trastornos hematológicos, como la anemia aplásica y ciertos trastornos genéticos, también requieren el TPH para el manejo de la enfermedad.
- A medida que aumenta el número de pacientes sometidos a TPH, también aumenta la demanda de un tratamiento eficaz para la EICH. Esta complicación frecuente del TPH se produce cuando las células del donante trasplantadas atacan los tejidos del receptor, lo que provoca síntomas potencialmente graves y potencialmente mortales. Por lo tanto, el aumento de la incidencia del TPH se traduce directamente en una mayor necesidad de tratamiento para la EICH para controlar y mitigar los efectos de esta complicación.
Por ejemplo,
- En octubre de 2023, según un artículo publicado por Frontiers Media SA, el número de trasplantes está aumentando constantemente a nivel mundial, con casi 20.000 trasplantes alo-HCT informados por la Sociedad Europea de Trasplante de Sangre y Médula Ósea (EBMT) en 2019 y más de 9.000 trasplantes en los Estados Unidos durante el mismo período, según el Centro para la Investigación Internacional de Trasplante de Sangre y Médula Ósea (CIBMTR).
Dinámica del mercado global del tratamiento de la enfermedad de injerto contra huésped (EICH)
Conductor
Campañas de concientización y educación del paciente en aumento
- Las campañas de concienciación y la educación del paciente son fundamentales para la detección temprana y el tratamiento de la enfermedad de injerto contra huésped (EICH). Dirigidas específicamente a profesionales sanitarios, pacientes y cuidadores, estas iniciativas resaltan los síntomas clave de la EICH, lo que permite un reconocimiento rápido y una intervención oportuna.
- Los pacientes y cuidadores bien informados tienen mayor probabilidad de buscar atención médica temprana, lo que previene que la enfermedad avance a etapas graves. Además, los profesionales de la salud, con un profundo conocimiento de los signos y el manejo de la EICH, pueden ofrecer una atención más eficaz, mejorando así los resultados del paciente. Estos esfuerzos específicos son fundamentales para mejorar la calidad de vida de las personas afectadas por la EICH.
Por ejemplo,
- En agosto de 2023, según un artículo publicado por Fierce Pharma, la leyenda del fútbol Mia Hamm se unió a Incyte para concienciar sobre la enfermedad de injerto contra huésped (EICH). Hamm comparte su experiencia personal tras perder a su hermano Garrett en 1997 debido a complicaciones tras un trasplante de médula ósea (TMO). Los TMO, destinados a curar enfermedades raras de la médula ósea, conllevan un riesgo importante de EICH, donde las células inmunitarias del injerto atacan a las células del receptor. Esta afección puede ser difícil de controlar después del trasplante y, en algunos casos, puede ser mortal.
- En febrero de 2023, PR Newswire informó que en EE. UU., la recién formada Alianza de la EICH, compuesta por organizaciones líderes en trasplantes, busca mejorar el acceso a recursos y el apoyo para las personas afectadas por la enfermedad de injerto contra huésped (EICH). Esta alianza designó el 17 de febrero como el Día de la EICH para concienciar y apoyar a la comunidad con EICH. Entre las organizaciones miembros se encuentran la Sociedad Americana de Trasplante y Terapia Celular (ASTCT), Be The Match (gestionada por el Programa Nacional de Donantes de Médula Ósea), la Red de Información sobre Trasplantes de Sangre y Médula Ósea (BMT InfoNet), la Fundación Meredith A. Cowden y el Enlace Nacional de Trasplante de Médula Ósea (nbmtLINK). La alianza busca conectar a los pacientes con grupos de apoyo y recursos, como trabajadores sociales y orientadores de pacientes, para ayudarlos a abogar por el tratamiento y abordar sus inquietudes.
Oportunidad
Expansión del mercado mediante iniciativas estratégicas y alianzas
- La expansión del mercado a través de la colaboración estratégica puede ser un enfoque muy eficaz para las empresas que operan en el mercado de tratamiento de la EICH (enfermedad de injerto contra huésped).
- A medida que aumenta la demanda de tratamientos innovadores y soluciones integrales para la EICH, las empresas pueden aprovechar las alianzas y colaboraciones estratégicas para expandir su presencia en el mercado y ofrecer una cartera de productos diversa y adaptada a las necesidades cambiantes de los pacientes. Al colaborar con instituciones de investigación, centros académicos y empresas biotecnológicas, los actores del mercado de la EICH pueden acceder a tecnologías de vanguardia, nuevos enfoques terapéuticos y experiencia científica para impulsar sus iniciativas de desarrollo de productos.
- Estas colaboraciones permiten a las empresas acelerar el descubrimiento y desarrollo de terapias prometedoras para la EICH, incluidas terapias celulares personalizadas, agentes biológicos específicos e intervenciones de cuidados de apoyo.
- Además, las alianzas estratégicas con líderes de opinión clave y grupos de defensa de los pacientes facilitan el acceso al mercado y mejoran la adopción de nuevos tratamientos al fomentar la confianza, la credibilidad y la participación de los pacientes.
- En marzo de 2021, según un artículo publicado por Bristol Myers Squibb, Bristol Myers Squibb (BMS) y Bluebird Bio unieron fuerzas en una alianza estratégica para desarrollar conjuntamente Abecma, una terapia celular inmunitaria personalizada, primera en su clase, dirigida por BCMA y administrada como una infusión única para pacientes con mieloma múltiple expuestos a triple clase. Se trata de una terapia de células T con receptor de antígeno del antígeno de maduración de células B (BCMA), diseñada específicamente para el tratamiento de la enfermedad de injerto contra huésped (EICH). Esta colaboración aprovecha la profunda experiencia de Bluebird Bio en terapia celular y la amplia capacidad de comercialización global de BMS. El objetivo es abordar las necesidades médicas no cubiertas de los pacientes con EICH en el futuro mediante el desarrollo de opciones de tratamiento innovadoras, allanando el camino para terapias pioneras en el campo de la EICH.
- En noviembre de 2022, un estudio de Labiotech UG reveló que Novartis se asoció con Gamida Cell para desarrollar y comercializar omidubicel, una terapia celular avanzada en investigación, para pacientes con neoplasias hematológicas de alto riesgo. Omidubicel está diseñado para mejorar los resultados del trasplante de médula ósea al ampliar la disponibilidad de células madre para trasplante. Esta colaboración refleja una iniciativa estratégica para abordar las necesidades no cubiertas en el campo de la oncología hematológica, con posibles aplicaciones para el tratamiento de la EICH.
- Además, la expansión del mercado a través de iniciativas estratégicas permite a los actores del mercado de EICH diversificar su cartera de productos y capturar una mayor participación en el mercado de EICH.
Restricción/Desafío
Regulaciones estrictas en las terapias para la EICH
- Las directrices regulatorias para la EICH son estrictas en comparación con las anteriores. Los fabricantes deben realizar cambios específicos en el producto antes de su aprobación, lo que previsiblemente causará retrasos.
- La estricta regulación representa un desafío significativo para el mercado de la EICH en el futuro. A medida que el campo avanza con el desarrollo de nuevas terapias y modalidades de tratamiento, las autoridades reguladoras imponen rigurosos estándares de seguridad, eficacia y control de calidad.
- Cumplir con estos requisitos regulatorios exige una inversión sustancial en desarrollo preclínico y clínico, así como una extensa documentación y presentación de datos para la aprobación regulatoria. Además, la complejidad de la EICH, su presentación heterogénea y la falta de criterios diagnósticos estandarizados complican aún más los procesos de evaluación y aprobación regulatoria.
- Por ejemplo,
- En abril de 2023, según un artículo publicado por la FDA, la investigación con células madre se legalizó en EE. UU. Sin embargo, existen ciertas restricciones relacionadas con su financiación. El Centro de Evaluación e Investigación Biológica (CBER) regula los productos de terapia celular, los productos de terapia génica humana y ciertos dispositivos relacionados con la terapia celular y génica. Si bien la FDA desempeña un papel crucial para garantizar la seguridad y la eficacia de las terapias, el proceso regulatorio puede ser riguroso y largo. Además, el cambiante panorama regulatorio de la FDA y los estrictos requisitos de vigilancia poscomercialización exigen esfuerzos continuos de cumplimiento y pueden afectar la velocidad con la que las nuevas terapias llegan al mercado.
- La regulación estricta actúa como un desafío importante para el mercado de EICH en el futuro, impactando los plazos de desarrollo de productos, los costos y el acceso al mercado.
Alcance del mercado europeo de tratamiento de la enfermedad de injerto contra huésped (EICH)
El mercado está segmentado según tipo, tipo de producto, sitio de absorción, grupo de edad, fuente, método de entrega, género y canal de distribución.
|
Segmentación |
Subsegmentación |
|
|
|
|
. |
|
|
|
|
|
|
|
|
|
Análisis regional del mercado europeo de tratamiento de la enfermedad de injerto contra huésped (EICH)
Alemania es la región dominante en el mercado del tratamiento de la enfermedad de injerto contra huésped (EICH)
- Alemania lidera el mercado europeo de tratamiento de la enfermedad de injerto contra huésped (EICH), impulsada por una alta prevalencia de obesidad, una infraestructura de atención médica avanzada y una sólida adopción de soluciones innovadoras para el control del peso.
- Alemania tiene una participación de mercado significativa debido a la creciente demanda de tratamientos farmacológicos como agonistas del receptor GLP-1, cirugías bariátricas y plataformas digitales de control de peso.
- Además, la creciente conciencia sobre los riesgos para la salud relacionados con la obesidad y la creciente adopción de programas de pérdida de peso impulsados por IA, consultas de telesalud y soluciones de tratamiento no invasivos continúan impulsando la expansión del mercado en la región.
Se proyecta que Alemania registre la mayor tasa de crecimiento.
- Se espera que Alemania sea testigo de la tasa de crecimiento más alta en el mercado europeo de tratamiento de la enfermedad de injerto contra huésped (EICH), impulsada por la creciente prevalencia de la enfermedad de injerto contra huésped (EICH), el aumento de las inversiones en atención médica y la creciente conciencia sobre las soluciones de control de peso.
- Países como ALEMANIA, Canadá y México están surgiendo como mercados clave debido a los cambios en los patrones de estilo de vida, la creciente urbanización y un número cada vez mayor de complicaciones de salud relacionadas con la obesidad.
- Alemania , con su creciente infraestructura sanitaria y la creciente demanda de tratamientos médicos para la pérdida de peso, sigue siendo un mercado crucial para el tratamiento de la enfermedad de injerto contra huésped (EICH). El país está experimentando una creciente adopción de tratamientos farmacológicos, cirugías bariátricas y programas digitales de control de peso.
Cuota de mercado del tratamiento de la enfermedad de injerto contra huésped (EICH) en Europa
El panorama competitivo del mercado ofrece detalles por competidor. Se incluye información general de la empresa, sus estados financieros, ingresos generados, potencial de mercado, inversión en investigación y desarrollo, nuevas iniciativas de mercado, presencia en Europa, plantas de producción, capacidad de producción, fortalezas y debilidades de la empresa, lanzamiento de productos, alcance y variedad de productos, y dominio de las aplicaciones. Los datos anteriores se refieren únicamente al enfoque de mercado de las empresas.
Los principales líderes del mercado que operan en el mercado son:
- Bristol-Myers Squibb Company (EE. UU.)
- AbbVie Inc. (EE. UU.)
- Novartis AG (Suiza)
- Janssen Asia-Pacific Services, LLC (EE. UU.)
- Mallinckrodt (Estados Unidos)
- Incyte (EE. UU.)
- Sanofi (Francia)
- Laboratorios Alkem Ltd. (India)
Últimos avances en el mercado europeo de tratamiento de la enfermedad de injerto contra huésped (EICH)
- En febrero de 2025, la FDA de ALEMANIA aprobó EMBLAVEO (aztreonam y avibactam), la primera combinación de monobactam e inhibidor de β-lactamasa, para el tratamiento de infecciones intraabdominales complicadas (IIAc) en adultos con opciones de tratamiento limitadas. Está dirigido contra bacterias gramnegativas, incluidas las cepas resistentes. Esta aprobación aborda la creciente resistencia a los antimicrobianos, una importante amenaza para la salud en Europa.
- En febrero de 2024, Johnson & Johnson, junto con su socio Pharmacyclics LLC (una empresa de AbbVie), recibió la aprobación de la FDA para una etiqueta ampliada de IMBRUVICA (ibrutinib) en suspensión oral. Esta ampliación permite el tratamiento de pacientes adultos con LLC/LLP, WM y EICHc tras un tratamiento sistémico previo.
- En marzo de 2024, Johnson & Johnson anunció la exitosa adquisición de Ambrx Biopharma, Inc. Esta compañía biofarmacéutica en fase clínica posee una plataforma tecnológica de biología sintética patentada que se utiliza para diseñar y desarrollar conjugados anticuerpo-fármaco (ADC) de nueva generación. Esta adquisición ofrece a Johnson & Johnson una oportunidad única para crear, desarrollar y comercializar terapias oncológicas dirigidas.
- En febrero de 2024, AbbVie Inc. y Tentarix Biotherapeutics anunciaron una alianza para descubrir y desarrollar conjuntamente productos biológicos multiespecíficos con actividad condicional en los campos de la oncología y la inmunología. Esta colaboración aprovechará la amplia experiencia de AbbVie en estas áreas, junto con la plataforma patentada Tentacles de Tentarix.
- En septiembre de 2021, Sanofi cerró un acuerdo de fusión con Kadmon Holdings, Inc., una compañía biofarmacéutica centrada en el desarrollo y la comercialización de terapias innovadoras para enfermedades con importantes necesidades médicas no cubiertas. Esta adquisición se alinea con la estrategia de crecimiento de Sanofi para sus activos principales de Medicamentos Generales e incorporará próximamente Rezurock (belumosudil) a su cartera de trasplantes.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 DBMR MARKET POSITION GRID
2.9 VENDOR SHARE ANALYSIS
2.1 SECONDARY SOURCES
2.11 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTAL ANALYSIS
4.2 PORTERS FIVE FORCES ANALYSIS
5 REGULATORY FRAMEWORK
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASING INCIDENCE OF HEMATOPOIETIC STEM CELL TRANSPLANTS (HSCT)
6.1.2 RISING AWARENESS CAMPAIGNS AND PATIENT EDUCATION
6.1.3 ADVANCEMENTS IN TREATMENT OPTIONS FOR GRAFT-VERSUS-HOST DISEASE (GVHD)
6.2 RESTRAINTS
6.2.1 HIGH COST OF MEDICATIONS AND SUPPORTIVE CARE
6.2.2 DISEASE HETEROGENEITY IN GRAFT-VERSUS-HOST DISEASE (GVHD) IMPLICATIONS FOR TREATMENT AND CLINICAL TRIALS
6.3 OPPORTUNITIES
6.3.1 MARKET EXPANSION THROUGH STRATEGIC INITIATIVES AND PARTNERSHIP
6.3.2 GROWING PIPELINE OF INNOVATIVE DRUGS FOR GVHD TREATMENT
6.3.3 EMPOWERING PATIENT-CENTRIC APPROACH TO GVHD TREATMENT
6.4 CHALLENGES
6.4.1 STRINGENT REGULATIONS IN GVHD THERAPIES
6.4.2 SAFETY CONCERN AND COMPLEXITIES DURING TREATMENT
7 EUROPE GRAFT-VERSUS-HOST-DISEASE (GVHD) TREATMENT MARKET, BY TYPE
7.1 OVERVIEW
7.2 CHRONIC GVHD
7.2.1 CORTICOSTEROIDS
7.2.1.1 PREDNISOLONE
7.2.1.2 METHYLPREDNISOLONE
7.2.2 IMMUNOSUPPRESSIVE
7.2.2.1 MYCOPHENOLATE MOFETIL (MMF)
7.2.2.2 METHOTREXATE (MTX)
7.2.2.3 ANTITHYMOCYTE GLOBULIN (ATG)
7.2.2.4 OTHERS
7.2.3 CALCINEURIN INHIBITORS
7.2.4 OTHERS
7.2.5 BRANDED
7.2.5.1 REZUROCK
7.2.5.2 IMBRUVICA
7.2.5.3 JAKAFI
7.2.6 GENERIC
7.3 ACUTE GVHD
7.3.1 CORTICOSTEROIDS
7.3.1.1 METHYLPREDNISOLONE
7.3.1.2 PREDNISOLONE
7.3.2 IMMUNOSUPPRESSIVE
7.3.2.1 MYCOPHENOLATE MOFETIL (MMF)
7.3.2.2 RUXOLITINIB
7.3.2.3 OTHERS
7.3.3 CALCINEURIN INHIBITORS
7.3.3.1 TACROLIMUS (TAC)
7.3.3.2 CYCLOSPORIN (CSA)
7.3.4 OTHERS
7.4 PROPHYLACTIC
7.4.1 CYCLOSPORIN (CSA)
7.4.2 METHOTREXATE (MTX)
7.4.3 TACROLIMUS (TAC)
7.4.4 METHYLPREDNISOLONE
7.4.5 OTHERS
7.4.6 GENERIC
7.4.7 BRANDED
8 EUROPE GRAFT-VERSUS-HOST- DISEASE (GVHD) TREATMENT MARKET, BY AGE
8.1 OVERVIEW
8.2 ADULTS
8.3 PEDIATRIC
9 EUROPE GRAFT-VERSUS-HOST- DISEASE (GVHD) TREATMENT MARKET, BY GENDER
9.1 OVERVIEW
9.2 FEMALE
9.3 MALE
10 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY METHOD OF ADMINISTRATION
10.1 OVERVIEW
10.2 ORAL
10.3 INTRAVENOUS
10.4 TOPICAL
10.5 OTHERS
11 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TREATMENT
11.1 OVERVIEW
11.2 MEDICATION
11.2.1 CORTICOSTEROIDS
11.2.1.1 METHYLPREDNISOLONE
11.2.1.2 PREDNISOLONE
11.2.2 IMMUNOSUPPRESSIVE
11.2.2.1 MYCOPHENOLATE MOFETIL (MMF)
11.2.2.2 METHOTREXATE (MTX)
11.2.2.3 ANTITHYMOCYTE GLOBULIN (ATG)
11.2.2.4 OTHERS
11.2.3 CALCINEURIN INHIBITORS
11.2.3.1 TACROLIMUS (TAC)
11.2.3.2 CYCLOSPORIN (CSA)
11.2.3.3 OTHERS
11.2.4 BRANDED
11.2.4.1 REZUROCK
11.2.4.2 IMBRUVICA
11.2.4.3 JAKAFI
11.2.4.4 ORENCIA
11.2.4.5 OTHERS
11.2.5 GENERIC
11.3 THERAPY
12 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS
12.2.1 PUBLIC
12.2.2 PRIVATE
12.3 TRANSPLANT CENTERS
12.4 INSTITUTES
12.5 SPECIALITY CENTERS
13 EUROPE GRAFT-VERSUS-HOST-DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 RETAIL SALES
13.3.1 HOSPITAL PHARMACY
13.3.2 RETAIL PHARMACY
13.3.3 ONLINE PHARMACY
13.4 OTHERS
14 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY REGION
14.1 EUROPE
14.1.1 GERMANY
14.1.2 FRANCE
14.1.3 ITALY
14.1.4 U.K.
14.1.5 SPAIN
14.1.6 BELGIUM
14.1.7 RUSSIA
14.1.8 SWITZERLAND
14.1.9 DENMARK
14.1.10 REST OF EUROPE
15 EUROPE GRAFT VERSUS HOST DISEASE (GVHD) TREATMENT MARKET: COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: GLOBAL
16 SWOT ANALYSIS
17 COMPANY PROFILE
17.1 BRISTOL-MYERS SQUIBB COMPANY
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENTS
17.2 ABBVIE INC.
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENTS
17.3 JANSSEN EUROPE SERVICES, LLC
17.3.1 COMPANY SNAPSHOT
17.3.2 REVENUE ANALYSIS
17.3.3 COMPANY SHARE ANALYSIS
17.3.4 PRODUCT PORTFOLIO
17.3.5 RECENT DEVELOPMENTS
17.4 SANOFI
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENTS/NEWS
17.5 INCYTE
17.5.1 COMPANY SNAPSHOT
17.5.2 REVENUE ANALYSIS
17.5.3 COMPANY SHARE ANALYSIS
17.5.4 PRODUCT PORTFOLIO
17.5.5 RECENT DEVELOPMENTS
17.6 ALKEM LABORATORIES LTD.
17.6.1 COMPANY SNAPSHOT
17.6.2 REVENUE ANALYSIS
17.6.3 PRODUCT PORTFOLIO
17.6.4 RECENT DEVELOPMENT
17.7 ASTELLAS PHARMA INC.
17.7.1 COMPANY SNAPSHOT
17.7.2 REVENUE ANALYSIS
17.7.3 PRODUCT PORTFOLIO
17.7.4 RECENT DEVELOPMENT
17.8 MALLINCKRODT
17.8.1 COMPANY SNAPSHOT
17.8.2 REVENUE ANALYSIS
17.8.3 PRODUCT PORTFOLIO
17.8.4 RECENT DEVELOPMENTS/NEWS
17.9 NOVARTIS AG
17.9.1 COMPANY SNAPSHOT
17.9.2 REVENUE ANALYSIS
17.9.3 PRODUCT PORTFOLIO
17.9.4 RECENT DEVELOPMENTS
18 QUESTIONNAIRE
19 RELATED REPORTS
Lista de Tablas
TABLE 1 EUROPE GRAFT-VERSUS-HOST- DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 2 EUROPE CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 3 EUROPE CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 4 EUROPE CORTICOSTEROIDS IN GRAFT-VERSUS-HOST- DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 5 EUROPE IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 6 EUROPE CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 7 EUROPE BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 8 EUROPE ACUTE GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 9 EUROPE ACUTE GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 10 EUROPE CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 11 EUROPE IMMUNOSUPPRESSIVES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 12 EUROPE CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 13 EUROPE PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 14 EUROPE PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 15 EUROPE PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 16 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY AGE, 2018-2032 (USD THOUSAND)
TABLE 17 EUROPE ADULTS IN GRAFT-VERSUS-HOST- DISEASE (GVHD) TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 18 EUROPE PEDIATRIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 19 EUROPE GRAFT-VERSUS-HOST- DISEASE (GVHD) TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 20 EUROPE FEMALE IN GRAFT-VERSUS-HOST- DISEASE (GVHD) TREATMENT MARKET, BY REGION, 20218-2032 (USD THOUSAND)
TABLE 21 EUROPE MALE IN GRAFT-VERSUS-HOST- DISEASE (GVHD) TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 22 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY METHOD OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 23 EUROPE ORAL IN GRAFT-VERSUS-HOST- DISEASE (GVHD) TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 24 EUROPE INTRAVENOUS IN GRAFT-VERSUS-HOST- DISEASE (GVHD) TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 25 EUROPE TOPICAL IN GRAFT-VERSUS-HOST- DISEASE (GVHD) TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 26 EUROPE OTHERS IN GRAFT-VERSUS-HOST- DISEASE (GVHD) TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 27 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 28 EUROPE MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 29 EUROPE MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 30 EUROPE CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 31 EUROPE IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 32 EUROPE CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 33 EUROPE MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 34 EUROPE BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 35 EUROPE THERAPY IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 36 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 37 EUROPE HOSPITALS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 38 EUROPE HOSPITALS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 39 EUROPE TRANSPLANT CENTERS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 40 EUROPE INSTITUTES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 41 EUROPE SPECIALTY CENTERS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 42 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 43 EUROPE DIRECT TENDER IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 44 EUROPE RETAIL SALES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 45 EUROPE RETAIL SALES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 46 EUROPE OTHERS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 47 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 48 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 49 EUROPE MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 50 EUROPE CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 51 EUROPE IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 52 EUROPE CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 53 EUROPE MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 54 EUROPE BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 55 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 56 EUROPE CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 57 METHYLPREDNISOLONE
TABLE 58 EUROPE IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 59 EUROPE CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 60 EUROPE BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 61 EUROPE ACUTE GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 62 EUROPE CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 63 EUROPE IMMUNOSUPPRESSIVES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 64 EUROPE CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 65 EUROPE PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 66 EUROPE PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 67 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 68 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY AGE, 2018-2032 (USD THOUSAND)
TABLE 69 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY METHOD OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 70 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 71 EUROPE HOSPITALS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 72 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 73 EUROPE RETAIL SALES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 74 GERMANY GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 75 GERMANY MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 76 GERMANY CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 77 GERMANY IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 78 GERMANY CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 79 GERMANY MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 80 GERMANY BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 81 GERMANY GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 82 GERMANY CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 83 GERMANY CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 84 GERMANY IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 85 GERMANY CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 86 GERMANY BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 87 GERMANY ACUTE GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 88 GERMANY CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 89 GERMANY IMMUNOSUPPRESSIVES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 90 GERMANY CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 91 GERMANY PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 92 GERMANY PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 93 GERMANY GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 94 GERMANY GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY AGE, 2018-2032 (USD THOUSAND)
TABLE 95 GERMANY GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY METHOD OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 96 GERMANY GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 97 GERMANY HOSPITALS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 98 GERMANY GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 99 GERMANY RETAIL SALES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 100 FRANCE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 101 FRANCE MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 102 FRANCE CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 103 FRANCE IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 104 FRANCE CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 105 FRANCE MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 106 FRANCE BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 107 FRANCE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 108 FRANCE CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 109 FRANCE CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 110 FRANCE IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 111 FRANCE CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 112 FRANCE BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 113 FRANCE ACUTE GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 114 FRANCE CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 115 FRANCE IMMUNOSUPPRESSIVES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 116 FRANCE CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 117 FRANCE PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 118 FRANCE PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 119 FRANCE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 120 FRANCE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY AGE, 2018-2032 (USD THOUSAND)
TABLE 121 FRANCE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY METHOD OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 122 FRANCE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 123 FRANCE HOSPITALS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 124 FRANCE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 125 FRANCE RETAIL SALES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 126 ITALY GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 127 ITALY MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 128 ITALY CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 129 ITALY IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 130 ITALY CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 131 ITALY MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 132 ITALY BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 133 ITALY GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 134 ITALY CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 135 ITALY CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 136 ITALY IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 137 ITALY CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 138 ITALY BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 139 ITALY ACUTE GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 140 ITALY CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 141 ITALY IMMUNOSUPPRESSIVES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 142 ITALY CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 143 ITALY PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 144 ITALY PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 145 ITALY GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 146 ITALY GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY AGE, 2018-2032 (USD THOUSAND)
TABLE 147 ITALY GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY METHOD OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 148 ITALY GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 149 ITALY HOSPITALS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 150 ITALY GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 151 ITALY RETAIL SALES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 152 U.K. GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 153 U.K. MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 154 U.K. CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 155 U.K. IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 156 U.K. CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 157 U.K. MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 158 U.K. BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 159 U.K. GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 160 U.K. CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 161 U.K. CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 162 U.K. IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 163 U.K. CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 164 U.K. BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 165 U.K. ACUTE GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 166 U.K. CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 167 U.K. IMMUNOSUPPRESSIVES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 168 U.K. CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 169 U.K. PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 170 U.K. PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 171 U.K. GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 172 U.K. GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY AGE, 2018-2032 (USD THOUSAND)
TABLE 173 U.K. GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY METHOD OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 174 U.K. GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 175 U.K. HOSPITALS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 176 U.K. GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 177 U.K. RETAIL SALES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 178 SPAIN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 179 SPAIN MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 180 SPAIN CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 181 SPAIN IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 182 SPAIN CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 183 SPAIN MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 184 SPAIN BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 185 SPAIN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 186 SPAIN CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 187 SPAIN CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 188 SPAIN IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 189 SPAIN CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 190 SPAIN BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 191 SPAIN ACUTE GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 192 SPAIN CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 193 SPAIN IMMUNOSUPPRESSIVES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 194 SPAIN CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 195 SPAIN PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 196 SPAIN PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 197 SPAIN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 198 SPAIN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY AGE, 2018-2032 (USD THOUSAND)
TABLE 199 SPAIN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY METHOD OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 200 SPAIN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 201 SPAIN HOSPITALS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 202 SPAIN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 203 SPAIN RETAIL SALES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 204 BELGIUM GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 205 BELGIUM MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 206 BELGIUM CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 207 BELGIUM IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 208 BELGIUM CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 209 BELGIUM MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 210 BELGIUM BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 211 BELGIUM GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 212 BELGIUM CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 213 BELGIUM CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 214 BELGIUM IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 215 BELGIUM CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 216 BELGIUM BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 217 BELGIUM ACUTE GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 218 BELGIUM CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 219 BELGIUM IMMUNOSUPPRESSIVES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 220 BELGIUM CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 221 BELGIUM PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 222 BELGIUM PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 223 BELGIUM GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 224 BELGIUM GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY AGE, 2018-2032 (USD THOUSAND)
TABLE 225 BELGIUM GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY METHOD OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 226 BELGIUM GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 227 BELGIUM HOSPITALS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 228 BELGIUM GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 229 BELGIUM RETAIL SALES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 230 RUSSIA GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 231 RUSSIA MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 232 RUSSIA CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 233 RUSSIA IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 234 RUSSIA CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 235 RUSSIA MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 236 RUSSIA BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 237 RUSSIA GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 238 RUSSIA CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 239 RUSSIA CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 240 RUSSIA IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 241 RUSSIA CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 242 RUSSIA BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 243 RUSSIA ACUTE GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 244 RUSSIA CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 245 RUSSIA IMMUNOSUPPRESSIVES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 246 RUSSIA CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 247 RUSSIA PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 248 RUSSIA PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 249 RUSSIA GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 250 RUSSIA GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY AGE, 2018-2032 (USD THOUSAND)
TABLE 251 RUSSIA GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY METHOD OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 252 RUSSIA GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 253 RUSSIA HOSPITALS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 254 RUSSIA GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 255 RUSSIA RETAIL SALES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 256 SWITZERLAND GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 257 SWITZERLAND MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 258 SWITZERLAND CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 259 SWITZERLAND IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 260 SWITZERLAND CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 261 SWITZERLAND MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 262 SWITZERLAND BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 263 SWITZERLAND GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 264 SWITZERLAND CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 265 SWITZERLAND CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 266 SWITZERLAND IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 267 SWITZERLAND CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 268 SWITZERLAND BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 269 SWITZERLAND ACUTE GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 270 SWITZERLAND CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 271 SWITZERLAND IMMUNOSUPPRESSIVES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 272 SWITZERLAND CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 273 SWITZERLAND PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 274 SWITZERLAND PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 275 SWITZERLAND GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 276 SWITZERLAND GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY AGE, 2018-2032 (USD THOUSAND)
TABLE 277 SWITZERLAND GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY METHOD OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 278 SWITZERLAND GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 279 SWITZERLAND HOSPITALS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 280 SWITZERLAND GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 281 SWITZERLAND RETAIL SALES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 282 DENMARK GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 283 DENMARK MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 284 DENMARK CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 285 DENMARK IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 286 DENMARK CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 287 DENMARK MEDICATION IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 288 DENMARK BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 289 DENMARK GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 290 DENMARK CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 291 DENMARK CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 292 DENMARK IMMUNOSUPPRESSIVE IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 293 DENMARK CHRONIC GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 294 DENMARK BRANDED IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 295 DENMARK ACUTE GVHD IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 296 DENMARK CORTICOSTEROIDS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 297 DENMARK IMMUNOSUPPRESSIVES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 298 DENMARK CALCINEURIN INHIBITORS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 299 DENMARK PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUGS, 2018-2032 (USD THOUSAND)
TABLE 300 DENMARK PROPHYLACTIC IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 301 DENMARK GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 302 DENMARK GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY AGE, 2018-2032 (USD THOUSAND)
TABLE 303 DENMARK GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY METHOD OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 304 DENMARK GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 305 DENMARK HOSPITALS IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 306 DENMARK GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 307 DENMARK RETAIL SALES IN GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 308 REST OF EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
Lista de figuras
FIGURE 1 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET: SEGMENTATION
FIGURE 2 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET: DROC ANALYSIS
FIGURE 4 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET: DBMR MARKET POSITION GRID
FIGURE 9 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET: SEGMENTATION
FIGURE 11 THE GROWING NUMBER OF STEM CELL TRANSPLANTATIONS IS DRIVING THE GROWTH OF THE EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET FROM 2025 TO 2032
FIGURE 12 THE TREATMENT SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET IN 2025 AND 2032
FIGURE 13 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET: EXECUTIVE SUMMARY
FIGURE 14 STRATEGIC DECISIONS
FIGURE 15 TWO SEGMENTS COMPRISE THE EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET, BY TREATMENT
FIGURE 16 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET
FIGURE 17 EUROPE GRAFT-VERSUS-HOST-DISEASE (GVHD) TREATMENT MARKET: BY TYPE, 2024
FIGURE 18 EUROPE GRAFT-VERSUS-HOST-DISEASE (GVHD) TREATMENT MARKET: BY TYPE, 2025-2032 (USD THOUSAND)
FIGURE 19 EUROPE GRAFT-VERSUS-HOST-DISEASE (GVHD) TREATMENT MARKET: BY TYPE, CAGR (2025-2032)
FIGURE 20 EUROPE GRAFT-VERSUS-HOST-DISEASE (GVHD) TREATMENT MARKET: BY TYPE, LIFELINE CURVE
FIGURE 21 EUROPE GRAFT-VERSUS-HOST- DISEASE (GVHD) TREATMENT MARKET: BY AGE, 2024..
FIGURE 22 EUROPE GRAFT-VERSUS-HOST- DISEASE (GVHD) TREATMENT MARKET: BY AGE, 2025-2032 (USD THOUSAND)
FIGURE 23 EUROPE GRAFT-VERSUS-HOST- DISEASE (GVHD) TREATMENT MARKET: BY AGE, CAGR (2025-2032)
FIGURE 24 EUROPE GRAFT-VERSUS-HOST- DISEASE (GVHD) TREATMENT MARKET: BY AGE, LIFELINE CURVE
FIGURE 25 EUROPE GRAFT-VERSUS-HOST- DISEASE (GVHD) TREATMENT MARKET: BY GENDER, 2024
FIGURE 26 EUROPE GRAFT-VERSUS-HOST- DISEASE (GVHD) TREATMENT MARKET: BY GENDER, 2025-2032 (USD THOUSAND)
FIGURE 27 EUROPE GRAFT-VERSUS-HOST- DISEASE (GVHD) TREATMENT MARKET: BY GENDER, CAGR (2025-2032)
FIGURE 28 EUROPE GRAFT-VERSUS-HOST- DISEASE (GVHD) TREATMENT MARKET: BY GENDER, LIFELINE CURVE
FIGURE 29 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET: BY METHOD OF ADMINISTRATION, 2024
FIGURE 30 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET: BY METHOD OF ADMINISTRATION, 2025-2032 (USD THOUSAND)
FIGURE 31 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET: BY METHOD OF ADMINISTRATION, CAGR (2025-2032)
FIGURE 32 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET: BY METHOD OF ADMINISTRATION, LIFELINE CURVE
FIGURE 33 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET: BY TREATMENT, 2024
FIGURE 34 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET: BY TREATMENT, 2025-2032 (USD THOUSAND)
FIGURE 35 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET: BY TREATMENT, CAGR (2025-2032)
FIGURE 36 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET: BY TREATMENT, LIFELINE CURVE
FIGURE 37 EUROPE GRAFT-VERSUS-HOST-DISEASE (GVHD) TREATMENT MARKET: BY END USER 2024
FIGURE 38 EUROPE GRAFT-VERSUS-HOST-DISEASE (GVHD) TREATMENT MARKET: BY END USER, 2025-2032 (USD THOUSAND)
FIGURE 39 EUROPE GRAFT-VERSUS-HOST-DISEASE (GVHD) TREATMENT MARKET: END USER, CAGR (2025-2032)
FIGURE 40 EUROPE GRAFT-VERSUS-HOST-DISEASE (GVHD) TREATMENT MARKET: END USER, LIFELINE CURVE
FIGURE 41 EUROPE GRAFT-VERSUS-HOST-DISEASE (GVHD) TREATMENT MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 42 EUROPE GRAFT-VERSUS-HOST-DISEASE (GVHD) TREATMENT MARKET: BY DISTRIBUTION CHANNEL, 2025-2032 (USD THOUSAND)
FIGURE 43 EUROPE GRAFT-VERSUS-HOST-DISEASE (GVHD) TREATMENT MARKET: DISTRIBUTION CHANNEL, CAGR (2025-2032)
FIGURE 44 EUROPE GRAFT-VERSUS-HOST-DISEASE (GVHD) TREATMENT MARKET: DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 45 EUROPE GRAFT-VERSUS-HOST DISEASE (GVHD) TREATMENT MARKET: SNAPSHOT (2024)
FIGURE 46 EUROPE GRAFT VERSUS HOST DISEASE (GVHD) TREATMENT MARKET: COMPANY SHARE 2024 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.