Mercado europeo de dispositivos de asistencia ventricular izquierda (LVAD), por tipo de producto (bomba cardíaca, controlador, baterías, cables), terapia (terapia puente al trasplante (BTT), terapia de destino, terapia puente a la candidatura (BTC), terapia puente a la recuperación (BTR)), grupo de edad (adulto, pediátrico), indicación (insuficiencia cardíaca congestiva, cardiopatía congénita , miocarditis, paro cardíaco, arritmias familiares y arrítmicas, miocardiopatías, insuficiencia cardíaca avanzada, otras), generación (dispositivos de segunda generación, dispositivos de tercera generación, dispositivos de primera generación), durabilidad (largo plazo, plazo intermedio, corto plazo), diseño (axial, centrífugo), tipo de pulso (no pulsátil, pulsátil), usuario final (hospitales, laboratorios de cateterismo cardíaco, clínicas especializadas, otros), canal de distribución (licitación directa, ventas minoristas, otros), país (Alemania, Francia, Estados Unidos) Reino Unido, Italia, Rusia, España, Turquía, Países Bajos, Suiza, Bélgica, Irlanda, Resto de Europa) Tendencias de la industria y pronóstico hasta 2029.
Análisis y perspectivas del mercado : mercado europeo de dispositivos de asistencia ventricular izquierda (LVAD)
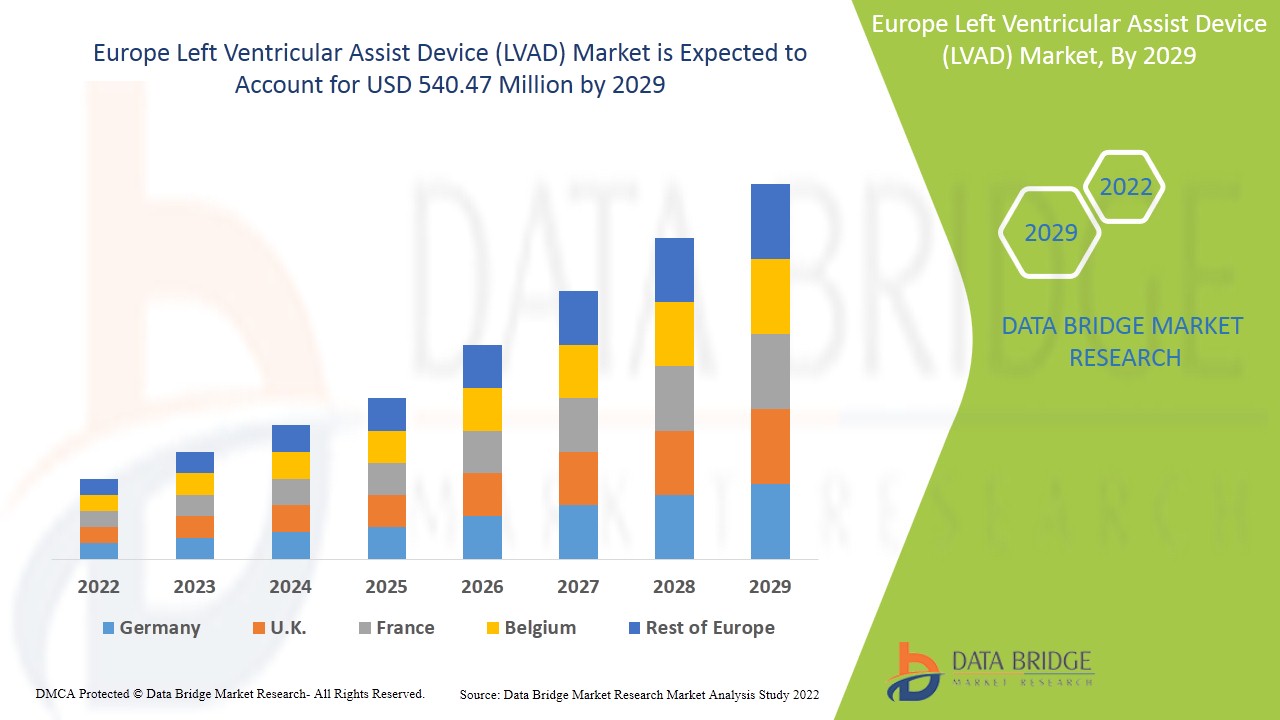
Se espera que el mercado europeo de dispositivos de asistencia ventricular izquierda (LVAD) gane crecimiento de mercado en el período de pronóstico de 2022 a 2029. Data Bridge Market Research analiza que el mercado está creciendo con una CAGR del 9,2% en el período de pronóstico de 2022 a 2029 y se espera que alcance los USD 540,47 millones para 2029. Los crecientes avances tecnológicos en el dispositivo de asistencia ventricular izquierda actúan como impulsor del crecimiento del mercado de dispositivos de asistencia ventricular izquierda (LVAD).
Un dispositivo de asistencia ventricular izquierda (LVAD, por sus siglas en inglés) es una bomba mecánica que se implanta en pacientes con insuficiencia cardíaca. Ayuda a la cámara inferior izquierda del corazón (ventrículo izquierdo) a bombear sangre desde el ventrículo hacia la aorta y el resto del cuerpo.
Se utiliza en pacientes que han llegado a una insuficiencia cardíaca terminal. El LVAD es una bomba mecánica que funciona con baterías y se implanta quirúrgicamente, y que ayuda al ventrículo izquierdo (la principal cámara de bombeo del corazón) a bombear sangre al resto del cuerpo.
Las principales razones que impulsan el crecimiento del mercado europeo de dispositivos de asistencia ventricular izquierda son el aumento del número de pacientes que sufren insuficiencia cardíaca y la escasez de donantes de corazón. Además, los dispositivos de asistencia ventricular izquierda técnicamente sofisticados (por ejemplo, HeartMate III) contribuyen al crecimiento del mercado. Además, se prevé que los avances actuales en esta disciplina, así como las nuevas aplicaciones de terapias innovadoras, abran nuevas vías para el mercado de los dispositivos de asistencia ventricular izquierda. No obstante, estos dispositivos son caros y plantean riesgos como coágulos sanguíneos y hemorragias, que se prevé que obstaculicen considerablemente la expansión del mercado. Además, el aumento de las retiradas de productos es un reto importante para el mercado.
El informe de mercado de dispositivos de asistencia ventricular izquierda (LVAD) proporciona detalles de la participación de mercado, nuevos desarrollos y análisis de la cartera de productos, el impacto de los actores del mercado nacional y localizado, analiza las oportunidades en términos de bolsillos de ingresos emergentes, cambios en las regulaciones del mercado, aprobaciones de productos, decisiones estratégicas, lanzamientos de productos, expansiones geográficas e innovaciones tecnológicas en el mercado. Para comprender el análisis y el escenario del mercado de dispositivos de asistencia ventricular izquierda (LVAD), comuníquese con Data Bridge Market Research para obtener un informe de analista; nuestro equipo lo ayudará a crear una solución de impacto en los ingresos para lograr su objetivo deseado.
Alcance y tamaño del mercado de dispositivos de asistencia ventricular izquierda (LVAD)
El mercado de dispositivos de asistencia ventricular izquierda (LVAD) está segmentado en función del tipo de producto, la terapia, el grupo de edad, la indicación, la generación, la durabilidad, el diseño, el tipo de pulso, el usuario final y el canal de distribución. El crecimiento entre segmentos le ayuda a analizar nichos de crecimiento y estrategias para abordar el mercado y determinar sus áreas de aplicación principales y la diferencia en sus mercados objetivo.
El mercado europeo de dispositivos de asistencia ventricular izquierda (LVAD) se clasifica en diez segmentos notables, como tipo de producto, terapia, grupo etario, indicación, generación, durabilidad, diseño, tipo de pulso, usuario final y canal de distribución.
- En función del tipo de producto, el mercado europeo de dispositivos de asistencia ventricular izquierda (LVAD) se segmenta en bomba cardíaca, controlador, baterías y cables. Las baterías se segmentan además en recargables y no recargables. En 2022, se espera que la bomba cardíaca domine el mercado, ya que realiza las funciones del corazón sin reemplazarlo y permite a los pacientes vivir más que aquellos que solo reciben tratamiento médico.
- Según la terapia, el mercado europeo de dispositivos de asistencia ventricular izquierda (LVAD) está segmentado en terapia puente al trasplante (BTT), terapia de destino, terapia puente a la candidatura (BTC) y terapia puente a la recuperación (BTR). En 2022, se prevé que el sector puente al trasplante (BTT) domine el mercado porque ayuda a los pacientes a esperar un trasplante y evita mayores daños al corazón y a otros órganos hasta que haya un donante disponible.
- Según el grupo de edad, el mercado europeo de dispositivos de asistencia ventricular izquierda (LVAD) se segmenta en adultos y niños. El segmento de adultos se segmenta a su vez en 19-39 años, 40-59 años, 60-79 años y mayores de 80 años. En 2022, se espera que el segmento de adultos domine el mercado, ya que la mayoría de los eventos de insuficiencia cardíaca ocurren en adultos. La prevalencia es del 1-2% de la población europea, aumentando a más del 10% entre las personas mayores de 70 años. Esto podría implicar que más de 10 millones de personas padecen insuficiencia cardíaca.
- Según la indicación, el mercado europeo de dispositivos de asistencia ventricular izquierda (LVAD) está segmentado en insuficiencia cardíaca congestiva, cardiopatía congénita, miocarditis, paro cardíaco, arritmias familiares y miocardiopatías arrítmicas, insuficiencia cardíaca avanzada y otras. En 2022, se espera que la insuficiencia cardíaca congestiva domine el mercado, ya que es el diagnóstico más común en pacientes hospitalizados mayores de 65 años. En Europa, más de 16 millones de personas han sido diagnosticadas con ICC.
- Según la generación, el mercado europeo de dispositivos de asistencia ventricular izquierda (LVAD) se segmenta en dispositivos de segunda generación, dispositivos de tercera generación y dispositivos de primera generación. En 2022, el mercado está dominado por las bombas rotativas de segunda generación, que tienen la ventaja de un diseño más pequeño y el potencial de una mayor confiabilidad mecánica a largo plazo al eliminar la cámara de depósito y las válvulas necesarias.
- En función de la durabilidad, el mercado europeo de dispositivos de asistencia ventricular izquierda (LVAD) se segmenta en largo plazo, mediano plazo y corto plazo. En 2022, se espera que el segmento de largo plazo domine el mercado debido a la necesidad de seguir respaldando las bombas cardíacas durante más tiempo, lo que está impulsando el crecimiento del mercado.
- Según el diseño, el mercado europeo de dispositivos de asistencia ventricular izquierda (LVAD) está segmentado en axial y centrífugo. En 2022, se espera que el segmento axial domine, ya que funciona de manera eficiente a altas velocidades de rotación y su precarga reducida.
- Según el tipo de pulso, el mercado europeo de dispositivos de asistencia ventricular izquierda (LVAD) se segmenta en no pulsátiles y pulsátiles. En 2022, se espera que el segmento de la categoría no pulsátil domine el mercado porque los LVAD se están utilizando con mayor frecuencia en terapias de destino; cualquier efecto adverso de una pulsatilidad arterial inadecuada en las estructuras periféricas podría verse enormemente magnificado con los largos períodos de implantación asociados con este tipo de terapia.
- Según el usuario final, el mercado europeo de dispositivos de asistencia ventricular izquierda (LVAD) está segmentado en hospitales, clínicas especializadas, laboratorios de cateterismo cardíaco y otros. En 2022, se espera que el segmento del sector hospitalario domine debido a un grupo creciente de pacientes cardiovasculares como resultado de los cambios en los estilos de vida y el envejecimiento de la población. En 2020, había más de 19.000 hospitales en 23 países europeos.
- Según el canal de distribución, el mercado europeo de dispositivos de asistencia ventricular izquierda (LVAD) se segmenta en licitación directa, ventas minoristas y otros. En 2022, se espera que el sector de licitación directa domine porque ofrece responsabilidad y productos de mayor calidad debido a la competencia, lo que aumenta la demanda de estos artículos.
Análisis a nivel de país del mercado de dispositivos de asistencia ventricular izquierda (LVAD)
Se analiza el mercado de dispositivos de asistencia ventricular izquierda (LVAD) y se proporciona información sobre el tamaño del mercado por país, diez segmentos notables como tipo de producto, terapia, grupo etario, indicación, generación, durabilidad, diseño, tipo de pulso, usuario final y canal de distribución como se mencionó anteriormente.
Los países cubiertos en el informe del mercado de dispositivos de asistencia ventricular izquierda (LVAD) de Europa son Alemania, Francia, Reino Unido, Italia, Rusia, España, Turquía, Países Bajos, Suiza, Bélgica, Irlanda y el resto de Europa.
Se espera que el segmento de adultos en Alemania crezca con la tasa de crecimiento más alta en el período de pronóstico de 2022 a 2029 debido a la creciente prevalencia de enfermedades cardiovasculares, incluida la insuficiencia cardíaca avanzada, el paro cardíaco y la insuficiencia cardíaca congestiva en adultos.
La sección de países del informe también proporciona factores de impacto de mercado individuales y cambios en la regulación en el mercado a nivel nacional que afectan las tendencias actuales y futuras del mercado. Los puntos de datos como nuevas ventas, ventas de reemplazo, demografía del país, leyes regulatorias y aranceles de importación y exportación son algunos de los principales indicadores utilizados para pronosticar el escenario del mercado para países individuales. Además, la presencia y disponibilidad de marcas europeas y sus desafíos enfrentados debido a la competencia grande o escasa de las marcas locales y nacionales, el impacto de los canales de venta se consideran al proporcionar un análisis de pronóstico de los datos del país.
Las crecientes actividades estratégicas de los principales actores del mercado para mejorar el conocimiento sobre el dispositivo de asistencia ventricular izquierda están impulsando el crecimiento del mercado del dispositivo de asistencia ventricular izquierda (LVAD)
El mercado de dispositivos de asistencia ventricular izquierda (LVAD) también le proporciona un análisis detallado del mercado para el crecimiento de cada país en un mercado en particular. Además, proporciona información detallada sobre la estrategia de los actores del mercado y su presencia geográfica. Los datos están disponibles para el período histórico de 2011 a 2020.
Análisis del panorama competitivo y de la cuota de mercado de los dispositivos de asistencia ventricular izquierda (LVAD)
El panorama competitivo del mercado de dispositivos de asistencia ventricular izquierda (LVAD) proporciona detalles por competidor. Los detalles incluidos son una descripción general de la empresa, las finanzas de la empresa, los ingresos generados, el potencial de mercado, la inversión en investigación y desarrollo, las nuevas iniciativas de mercado, los sitios e instalaciones de producción, las fortalezas y debilidades de la empresa, el lanzamiento de productos, las líneas de prueba de productos, las aprobaciones de productos, las patentes, la amplitud y la extensión de los productos, el dominio de las aplicaciones, la curva de la línea de vida de la tecnología. Los puntos de datos anteriores proporcionados solo están relacionados con el enfoque de la empresa relacionado con el mercado de dispositivos de asistencia ventricular izquierda (LVAD).
Las principales empresas que se dedican al dispositivo de asistencia ventricular izquierda (LVAD) son ABIOMED, Abbott, Evaheart, Inc, Saft, Berlin Heart, CorWave SA, Jarvik Heart, Inc. y otras empresas nacionales. Los analistas de DBMR comprenden las fortalezas competitivas y brindan un análisis competitivo para cada competidor por separado.
Numerosas empresas de todo el mundo también han iniciado numerosos contratos y acuerdos que están acelerando también el mercado de dispositivos de asistencia ventricular izquierda (LVAD).
Por ejemplo,
- En enero de 2021, CorWave SA recibió una financiación de 40 millones de dólares de tres inversores para desarrollar una bomba cardíaca de membrana de onda avanzada. La financiación recibida permitió a la empresa acelerar el desarrollo del producto y fortalecer su presencia en el mercado mundial de dispositivos de asistencia ventricular izquierda (LVAD).
La colaboración, el lanzamiento de productos, la expansión comercial, los premios y reconocimientos, las empresas conjuntas y otras estrategias del actor del mercado están mejorando la presencia de la empresa en el mercado de dispositivos de asistencia ventricular izquierda (LVAD), lo que también brinda beneficios para el crecimiento de las ganancias de la organización.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL
4.2 PORTER'S
4.3 INDUSTRIAL INSIGHTS:
5 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: REGULATIONS
5.1 REGULATION IN U.S.
5.1.1 GUIDELINES-
5.2 REGULATION IN EUROPE
5.2.1 GUIDELINES FOR THE MANUFACTURERS:
5.3 REGULATION IN CANADA
5.3.1 GUIDELINES FOR THE MANUFACTURERS:
5.4 REGULATION IN MEXICO
5.4.1 GUIDELINES FOR THE MANUFACTURERS:
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASING GERIATRIC POPULATION ALONG WITH RISING PREVALENCE OF CARDIAC DISEASES
6.1.2 PRESENCE OF FAVOURABLE REIMBURSEMENT POLICIES
6.1.3 CHANGING LIFESTYLE TRIGGERS THE DEVELOPMENT OF CARDIOVASCULAR DISEASES
6.2 RESTRAINTS
6.2.1 HIGH COST OF LVAD IMPLANTATION AND TREATMENT
6.2.2 COMPLICATIONS AND RISKS ASSOCIATED WITH LVAD
6.3 OPPORTUNITIES
6.3.1 INCREASE IN HEALTHCARE EXPENDITURE
6.3.2 INCREASE IN MINIMALLY INVASIVE PROCEDURE
6.3.3 INCREASING SHORTAGE OF ORGAN DONORS
6.3.4 TECHNOLOGICAL ADVANCEMENTS IN LEFT VENTRICULAR ASSIST DEVICES
6.4 CHALLENGES
6.4.1 ONGOING COVID-19
6.4.2 INCREASE IN PRODUCT RECALL
7 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE
7.1 OVERVIEW
7.2 HEART PUMP
7.3 CONTROLLER
7.4 BATTERIES
7.4.1 RECHARGEABLE
7.4.2 NON-RECHARGEABLE
7.5 WIRES
8 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY
8.1 OVERVIEW
8.2 BRIDGE-TO-TRANSPLANT (BTT) THERAPY
8.2.1 HEART PUMP
8.2.2 CONTROLLER
8.2.3 BATTERIES
8.2.4 WIRES
8.3 DESTINATION THERAPY
8.3.1 HEART PUMP
8.3.2 CONTROLLER
8.3.3 BATTERIES
8.3.4 WIRES
8.4 BRIDGE-TO-CANDIDACY (BTC) THERAPY
8.4.1 HEART PUMP
8.4.2 CONTROLLER
8.4.3 BATTERIES
8.4.4 WIRES
8.5 BRIDGE-TO-RECOVERY (BTR) THERAPY
8.5.1 HEART PUMP
8.5.2 CONTROLLER
8.5.3 BATTERIES
8.5.4 WIRES
9 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP
9.1 OVERVIEW
9.2 ADULT
9.2.1 19-39 YEARS
9.2.2 40-59 YEARS
9.2.3 60-79 YEARS
9.2.4 ABOVE 80 YEARS
9.3 PEDIATRIC
10 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION
10.1 OVERVIEW
10.2 SECOND GENERATION DEVICES
10.3 THIRD GENERATION DEVICES
10.4 FIRST GENERATION DEVICES
11 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN
11.1 OVERVIEW
11.2 AXIAL
11.3 CENTRIFUGAL
12 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION
12.1 OVERVIEW
12.2 CONGESTIVE HEART FAILURE
12.2.1 HEART PUMP
12.2.2 CONTROLLER
12.2.3 BATTERIES
12.2.4 WIRES
12.3 CONGENITAL HEART DISEASE
12.3.1 HEART PUMP
12.3.2 CONTROLLER
12.3.3 BATTERIES
12.3.4 WIRES
12.4 MYOCARDITIS
12.4.1 HEART PUMP
12.4.2 CONTROLLER
12.4.3 BATTERIES
12.4.4 WIRES
12.5 CARDIAC ARREST
12.5.1 HEART PUMP
12.5.2 CONTROLLER
12.5.3 BATTERIES
12.5.4 WIRES
12.6 FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES
12.6.1 HEART PUMP
12.6.2 CONTROLLER
12.6.3 BATTERIES
12.6.4 WIRES
12.7 ADVANCED HEART FAILURE
12.7.1 HEART PUMP
12.7.2 CONTROLLER
12.7.3 BATTERIES
12.7.4 WIRES
12.8 OTHERS
13 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET, BY DURABILITY
13.1 OVERVIEW
13.2 LONG-TERM
13.3 INTERMEDIATE-TERM
13.4 SHORT-TERM
14 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET, BY PULSE TYPE
14.1 OVERVIEW
14.2 NONPULSATILE
14.3 PULSATILE
15 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET, BY END USER
15.1 OVERVIEW
15.2 HOSPITAL
15.3 CARDIAC CATH LABORATORIES
15.4 SPECIALTY CLINICS
15.5 OTHERS
16 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET, BY DISTRIBUTION CHANNEL
16.1 OVERVIEW
16.2 DIRECT TENDER
16.3 RETAIL SALES
16.4 OTHERS
17 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION
17.1 EUROPE
17.1.1 GERMANY
17.1.2 FRANCE
17.1.3 U.K.
17.1.4 ITALY
17.1.5 RUSSIA
17.1.6 SPAIN
17.1.7 TURKEY
17.1.8 NETHERLANDS
17.1.9 SWITZERLAND
17.1.10 BELGIUM
17.1.11 IRELAND
17.1.12 REST OF EUROPE
18 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: COMPANY LANDSCAPE
18.1 COMPANY SHARE ANALYSIS: EUROPE
19 SWOT ANALYSIS
20 COMPANY PROFILE
20.1 ABIOMED
20.1.1 COMPANY SNAPSHOT
20.1.2 REVENUE ANALYSIS
20.1.3 COMPANY SHARE ANALYSIS
20.1.4 PRODUCT PORTFOLIO
20.1.5 RECENT DEVELOPMENTS
20.1.5.1 FDA APPROVAL
20.1.5.2 FDA APPROVAL
20.2 ABBOTT
20.2.1 COMPANY SNAPSHOT
20.2.2 REVENUE ANALYSIS
20.2.3 COMPANY SHARE ANALYSIS
20.2.4 PRODUCT PORTFOLIO
20.2.5 RECENT DEVELOPMENTS
20.2.5.1 SUPPLY INCREMENT
20.2.5.2 BREAK THROUGH DEVICE DESIGNATION
20.3 BERLIN HEART
20.3.1 COMPANY SNAPSHOT
20.3.2 COMPANY SHARE ANALYSIS
20.3.3 PRODUCT PORTFOLIO
20.3.4 RECENT DEVELOPMENT
20.3.4.1 PRODUCT APPROVAL
20.4 SAFT (A SUBSIDIARY OF TOTALENERGIES)
20.4.1 COMPANY SNAPSHOT
20.4.2 REVENUE ANALYSIS
20.4.3 COMPANY SHARE ANALYSIS
20.4.4 PRODUCT PORTFOLIO
20.4.5 RECENT DEVELOPMENT
20.4.5.1 PARTNERSHIP
20.5 JARVIK HEART, INC.
20.5.1 COMPANY SNAPSHOT
20.5.2 COMPANY SHARE ANALYSIS
20.5.3 PRODUCT PORTFOLIO
20.5.4 RECENT DEVELOPMENT
20.5.4.1 FDA APPROVAL
20.6 CORWAVE SA
20.6.1 COMPANY SNAPSHOT
20.6.2 PRODUCT PORTFOLIO
20.6.3 RECENT DEVELOPMENT
20.6.3.1 EXPANSION
20.7 EVAHEART, INC
20.7.1 COMPANY SNAPSHOT
20.7.2 PRODUCT PORTFOLIO
20.7.3 RECENT DEVELOPMENT
20.7.3.1 PRODUCT TRIAL
21 QUESTIONNAIRE
22 RELATED REPORTS
Lista de Tablas
TABLE 1 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 2 EUROPE HEART PUMP IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 3 EUROPE CONTROLLER IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 EUROPE BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 EUROPE BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 6 EUROPE WIRES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 8 EUROPE BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 9 EUROPE BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 10 EUROPE DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 EUROPE DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 12 EUROPE BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 EUROPE BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 14 EUROPE BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 EUROPE BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 16 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 17 EUROPE ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 EUROPE ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 19 EUROPE PEDIATRIC IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 21 EUROPE SECOND GENERATION DEVICES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 EUROPE THIRD GENERATION DEVICES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 EUROPE FIRST GENERATION DEVICES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 25 EUROPE AXIAL IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 EUROPE CENTRIFUGAL IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 28 EUROPE CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 EUROPE CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 30 EUROPE CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 EUROPE CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 32 EUROPE MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 EUROPE MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 34 EUROPE CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 EUROPE CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 36 EUROPE FAMILIAL ARRHYTHYMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 37 EUROPE FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 38 EUROPE ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 39 EUROPE ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 40 EUROPE OTHERS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 41 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 42 EUROPE LONG TERM IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 43 EUROPE INTERMEDIATE-TERM IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 44 EUROPE SHORT-TERM IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION))
TABLE 45 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 46 EUROPE NONPULSATILE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 47 EUROPE PULSATILE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 48 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 49 EUROPE HOSPITALS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 50 EUROPE CARDIAC CATH LABORATORIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 51 EUROPE SPECIALTY CLINICS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 52 EUROPE OTHERS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 53 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 54 EUROPE DIRECT TENDER IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 55 EUROPE RETAIL SALES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 56 EUROPE OTHERS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 57 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
TABLE 58 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 59 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 60 EUROPE BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 61 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 62 EUROPE BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 63 EUROPE DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 64 EUROPE BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 65 EUROPE BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 66 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 67 EUROPE ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 68 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 69 EUROPE CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 70 EUROPE CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 71 EUROPE MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 72 EUROPE CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 73 EUROPE FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 74 EUROPE ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 75 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 76 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 77 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 78 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 79 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 80 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 81 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 82 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 83 GERMANY BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 84 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 85 GERMANY BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 86 GERMANY DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 87 GERMANY BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 88 GERMANY BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 89 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 90 GERMANY ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 91 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 92 GERMANY CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 93 GERMANY CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 94 GERMANY MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 95 GERMANY CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 96 GERMANY FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 97 GERMANY ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 98 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 99 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 100 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 101 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 102 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 103 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 104 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 105 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 106 FRANCE BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 107 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 108 FRANCE BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 109 FRANCE DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 110 FRANCE BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 111 FRANCE BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 112 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 113 FRANCE ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 114 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 115 FRANCE CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 116 FRANCE CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 117 FRANCE MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 118 FRANCE CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 119 FRANCE FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 120 FRANCE ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 121 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 122 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 123 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 124 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 125 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 126 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 127 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 128 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 129 U.K. BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 130 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 131 U.K. BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 132 U.K. DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 133 U.K. BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 134 U.K. BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 135 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 136 U.K. ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 137 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 138 U.K. CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 139 U.K. CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 140 U.K. MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 141 U.K. CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 142 U.K. FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 143 U.K. ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 144 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 145 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 146 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 147 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 148 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 149 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 150 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 151 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 152 ITALY BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 153 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 154 ITALY BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 155 ITALY DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 156 ITALY BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 157 ITALY BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 158 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 159 ITALY ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 160 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 161 ITALY CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 162 ITALY CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 163 ITALY MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 164 ITALY CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 165 ITALY FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 166 ITALY ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 167 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 168 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 169 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 170 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 171 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 172 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 173 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 174 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 175 RUSSIA BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 176 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 177 RUSSIA BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 178 RUSSIA DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 179 RUSSIA BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 180 RUSSIA BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 181 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 182 RUSSIA ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 183 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 184 RUSSIA CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 185 RUSSIA CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 186 RUSSIA MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 187 RUSSIA CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 188 RUSSIA FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 189 RUSSIA ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 190 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 191 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 192 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 193 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 194 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 195 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 196 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 197 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 198 SPAIN BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 199 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 200 SPAIN BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 201 SPAIN DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 202 SPAIN BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 203 SPAIN BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 204 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 205 SPAIN ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 206 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 207 SPAIN CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 208 SPAIN CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 209 SPAIN MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 210 SPAIN CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 211 SPAIN FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 212 SPAIN ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 213 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 214 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 215 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 216 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 217 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 218 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 219 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 220 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 221 TURKEY BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 222 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 223 TURKEY BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 224 TURKEY DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 225 TURKEY BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 226 TURKEY BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 227 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 228 TURKEY ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 229 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 230 TURKEY CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 231 TURKEY CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 232 TURKEY MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 233 TURKEY CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 234 TURKEY FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 235 TURKEY ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 236 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 237 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 238 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 239 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 240 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 241 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 242 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 243 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 244 NETHERLANDS BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 245 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 246 NETHERLANDS BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 247 NETHERLANDS DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 248 NETHERLANDS BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 249 NETHERLANDS BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 250 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 251 NETHERLANDS ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 252 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 253 NETHERLANDS CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 254 NETHERLANDS CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 255 NETHERLANDS MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 256 NETHERLANDS CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 257 NETHERLANDS FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 258 NETHERLANDS ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 259 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 260 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 261 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 262 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 263 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 264 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 265 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 266 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 267 SWITZERLAND BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 268 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 269 SWITZERLAND BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 270 SWITZERLAND DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 271 SWITZERLAND BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 272 SWITZERLAND BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 273 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 274 SWITZERLAND ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 275 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 276 SWITZERLAND CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 277 SWITZERLAND CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 278 SWITZERLAND MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 279 SWITZERLAND CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 280 SWITZERLAND FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 281 SWITZERLAND ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 282 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 283 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 284 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 285 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 286 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 287 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 288 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 289 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 290 BELGIUM BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 291 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 292 BELGIUM BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 293 BELGIUM DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 294 BELGIUM BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 295 BELGIUM BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 296 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 297 BELGIUM ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 298 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 299 BELGIUM CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 300 BELGIUM CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 301 BELGIUM MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 302 BELGIUM CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 303 BELGIUM FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 304 BELGIUM ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 305 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 306 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 307 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 308 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 309 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 310 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 311 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 312 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 313 IRELAND BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 314 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 315 IRELAND BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 316 IRELAND DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 317 IRELAND BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 318 IRELAND BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 319 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 320 IRELAND ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 321 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 322 IRELAND CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 323 IRELAND CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 324 IRELAND MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 325 IRELAND CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 326 IRELAND FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 327 IRELAND ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 328 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 329 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 330 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 331 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 332 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 333 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 334 REST OF EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
Lista de figuras
FIGURE 1 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: SEGMENTATION
FIGURE 2 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: DROC ANALYSIS
FIGURE 4 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: EUROPE VS REGIONAL ANALYSIS
FIGURE 5 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: END USER COVERAGE GRID
FIGURE 9 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET:VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: SEGMENTATION
FIGURE 11 INCREASING PREVALENCE OF CARDIOVASCULAR DISEASE AND RISING HEALTHCARE EXPENDITURE ARE EXPECTED TO DRIVE THE EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 HEART PUMP SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET IN 2022 & 2029
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET AND ASIA-PACIFIC TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET
FIGURE 15 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY PRODUCT TYPE, 2021
FIGURE 16 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY PRODUCT TYPE, 2020-2029 (USD MILLION)
FIGURE 17 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY PRODUCT TYPE, CAGR (2022-2029)
FIGURE 18 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 19 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY THERAPY, 2021
FIGURE 20 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY THERAPY, 2020-2029 (USD MILLION)
FIGURE 21 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY THERAPY, CAGR (2022-2029)
FIGURE 22 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY THERAPY, LIFELINE CURVE
FIGURE 23 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY AGE GROUP, 2021
FIGURE 24 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY AGE GROUP, 2020-2029 (USD MILLION)
FIGURE 25 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY AGE GROUP, CAGR (2022-2029)
FIGURE 26 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY AGE GROUP, LIFELINE CURVE
FIGURE 27 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY GENERATION, 2021
FIGURE 28 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY GENERATION, 2020-2029 (USD MILLION)
FIGURE 29 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY GENERATION, CAGR (2022-2029)
FIGURE 30 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY GENERATION, LIFELINE CURVE
FIGURE 31 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY DESIGN, 2021
FIGURE 32 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY DESIGN, 2020-2029 (USD MILLION)
FIGURE 33 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY DESIGN, CAGR (2022-2029)
FIGURE 34 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY DESIGN, LIFELINE CURVE
FIGURE 35 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY INDICATION, 2021
FIGURE 36 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY INDICATION, 2020-2029 (USD MILLION)
FIGURE 37 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY INDICATION, CAGR (2022-2029)
FIGURE 38 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY INDICATION, LIFELINE CURVE
FIGURE 39 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DURABILITY, 2021
FIGURE 40 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DURABILITY, 2020-2029 (USD MILLION)
FIGURE 41 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DURABILITY, CAGR (2022-2029)
FIGURE 42 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DURABILITY, LIFELINE CURVE
FIGURE 43 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY PULSE TYPE, 2021
FIGURE 44 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY PULSE TYPE, 2020-2029 (USD MILLION)
FIGURE 45 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY PULSE TYPE, CAGR (2022-2029)
FIGURE 46 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY PULSE TYPE, LIFELINE CURVE
FIGURE 47 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY END USER, 2021
FIGURE 48 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY END USER, 2020-2029 (USD MILLION)
FIGURE 49 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY END USER, CAGR (2022-2029)
FIGURE 50 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY END USER, LIFELINE CURVE
FIGURE 51 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 52 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
FIGURE 53 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 54 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 55 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: SNAPSHOT (2021)
FIGURE 56 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY COUNTRY (2021)
FIGURE 57 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY COUNTRY (2022 & 2029)
FIGURE 58 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY COUNTRY (2021 & 2029)
FIGURE 59 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY PRODUCT TYPE (2022-2029)
FIGURE 60 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: COMPANY SHARE 2021 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.