Europe Postpartum Hemorrhage Treatment Devices Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
274.95 Million
USD
397.15 Million
2024
2032
USD
274.95 Million
USD
397.15 Million
2024
2032
| 2025 –2032 | |
| USD 274.95 Million | |
| USD 397.15 Million | |
|
|
|
|
Mercado europeo de dispositivos para el tratamiento de hemorragias posparto, tipo (taponamiento uterino con balón, sistema de inyección precargado Uniject, prenda antichoque no neumática, dispositivos de control de hemorragias por vacío y otros), afección (hemorragia posparto mayor (más de 1000 ml), hemorragia posparto menor (500-1000 ml), hemorragia posparto masiva (2000 ml o más) y hemorragia posparto secundaria), tipo de paciente (HPP primaria y secundaria), usuario final (hospitales, centros de maternidad, clínicas especializadas, centros de atención domiciliaria y otros), canal de distribución (licitación directa, venta minorista y otros): tendencias del sector y pronóstico hasta 2032.
Tamaño del mercado de dispositivos para el tratamiento de hemorragias posparto
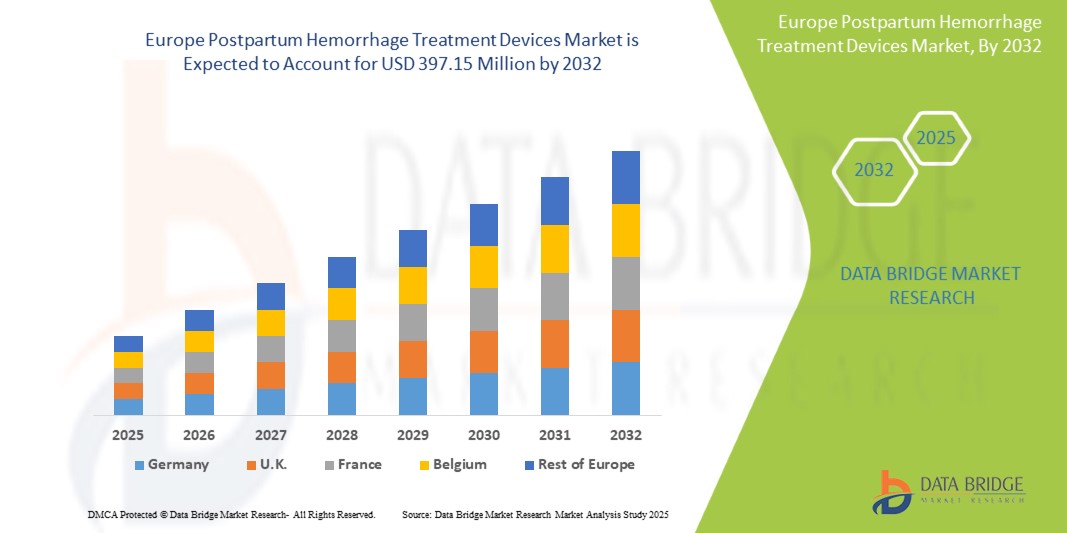
- El mercado europeo de dispositivos para el tratamiento de hemorragias posparto se valoró en 274,95 millones de dólares en 2024 y se espera que alcance los 397,15 millones de dólares en 2032.
- Durante el período de pronóstico de 2025 a 2032, es probable que el mercado crezca a una CAGR del 4,7 %, impulsado principalmente por la creciente incidencia de hemorragia posparto.
- Los impulsores clave del mercado de dispositivos para el tratamiento de hemorragias posparto incluyen la creciente incidencia de hemorragias posparto, la creciente conciencia sobre tratamientos efectivos y los avances en tecnología.
Análisis de dispositivos para el tratamiento de hemorragias posparto
- La creciente incidencia de hemorragia posparto, impulsada por factores como las crecientes tasas de cesáreas y complicaciones durante el parto, ha generado una creciente demanda de dispositivos de tratamiento eficaces.
- Las innovaciones en tecnología médica, incluidos los dispositivos de taponamiento uterino más nuevos, los agentes hemostáticos y las opciones quirúrgicas mínimamente invasivas, están contribuyendo a mejorar los resultados de los pacientes e impulsando el crecimiento del mercado.
- Por ejemplo, el mercado de dispositivos para el tratamiento de la HPP varía geográficamente, con un crecimiento significativo observado en las regiones en desarrollo debido al aumento de las inversiones en atención médica y el enfoque en la salud materna.
- Por lo tanto, el mercado de dispositivos para el tratamiento de la HPP muestra una fuerte disparidad geográfica, y las regiones en desarrollo experimentan un crecimiento significativo debido al aumento de las inversiones en atención médica y a un enfoque centrado en mejorar la salud materna.
Alcance del informe y segmentación de los dispositivos para el tratamiento de la hemorragia posparto
|
Atributos |
Dispositivos para el tratamiento de hemorragias posparto: Perspectivas clave del mercado |
|
Segmentos cubiertos |
|
|
Países cubiertos |
Europa
|
|
Actores clave del mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de información de datos de valor añadido |
Además de los conocimientos sobre escenarios de mercado como valor de mercado, tasa de crecimiento, segmentación, cobertura geográfica y actores principales, los informes de mercado seleccionados por Data Bridge Market Research también incluyen análisis de importación y exportación, descripción general de la capacidad de producción, análisis del consumo de producción, análisis de tendencias de precios, escenario de cambio climático, análisis de la cadena de suministro, análisis de la cadena de valor, descripción general de materias primas/consumibles, criterios de selección de proveedores, análisis PESTLE, análisis de Porter y marco regulatorio. |
Tendencias del mercado de dispositivos para el tratamiento de hemorragias posparto
Mayor adopción de dispositivos de taponamiento uterino con balón
- Los dispositivos de taponamiento uterino con balón están diseñados para controlar la HPP mediante presión directa sobre la pared uterina, lo que promueve la hemostasia. Su eficacia en entornos clínicos ha sido ampliamente documentada, lo que ha generado mayor confianza entre los profesionales de la salud en el uso de estos dispositivos como intervención de primera línea para el manejo de la HPP, impulsando así su adopción.
- Por ejemplo, se ha realizado un esfuerzo conjunto en la comunidad sanitaria para mejorar la formación y la educación sobre el manejo de la HPP. Esto incluye capacitación específica sobre el uso de técnicas de taponamiento uterino con balón. Una mayor concienciación entre los profesionales sanitarios sobre la importancia de una intervención rápida ha propiciado una mayor aceptación y utilización de estos dispositivos tanto en países desarrollados como en desarrollo.
- Los dispositivos de taponamiento uterino con balón se están integrando cada vez más en los protocolos integrales de atención materna, en particular en entornos obstétricos de emergencia.
- Esta integración está respaldada por las directrices de organizaciones como la OMS y diversas iniciativas de salud materna, que promueven prácticas basadas en la evidencia para prevenir y gestionar eficazmente la HPP. La alineación de estos dispositivos con las directrices clínicas está facilitando su adopción generalizada en hospitales y centros de maternidad.
Conductores
Aumento de la incidencia de hemorragia posparto
- La creciente incidencia de la hemorragia posparto (HPP) ha generado una necesidad apremiante de dispositivos de tratamiento eficaces en el mercado global. Una mayor concienciación sobre los problemas de salud materna y una mejor identificación de las poblaciones en riesgo han impulsado un aumento en la demanda de soluciones innovadoras.
- A medida que los sistemas de atención médica priorizan la seguridad materna, la adopción de dispositivos médicos avanzados destinados a prevenir o controlar la HPP está en aumento, incluidos dispositivos de compresión uterina y agentes hemostáticos.
Por ejemplo,
- En mayo de 2020, según el NCBI, la hemorragia posparto (HPP) fue la principal causa de mortalidad materna a nivel mundial. En EE. UU., la HPP aumentó un 26 %. Este alarmante aumento de casos resalta la importancia crucial de abordar la HPP y sirve como catalizador para una mayor inversión en soluciones y tecnologías de tratamiento innovadoras destinadas a prevenir y controlar esta afección potencialmente mortal.
- En agosto de 2024, la Organización Mundial de la Salud declaró que la hemorragia posparto (HPP), comúnmente definida como una pérdida de sangre de 500 ml o más en las 24 horas posteriores al parto, es la principal causa de mortalidad materna en todo el mundo. Afecta a millones de mujeres cada año y representa más del 20 % de todas las muertes maternas reportadas a nivel mundial.
Oportunidades
Programas de capacitación y educación para el uso adecuado de los dispositivos de tratamiento de la HPP
- El desarrollo de programas integrales de capacitación y educación para el uso adecuado de los dispositivos de tratamiento de la HPP (hemorragia posparto) representa una valiosa oportunidad para fortalecer los servicios de salud materna.
- Estos programas se pueden adaptar para trabajadores de la salud en distintos niveles, desde trabajadores de salud comunitarios hasta parteras calificadas y personal hospitalario, asegurando que todo el personal involucrado en la atención materna esté equipado con los conocimientos y la experiencia práctica necesarios.
Restricciones/Desafíos
Impacto ambiental y cuestiones de eliminación de dispositivos PPH de un solo uso
- Los dispositivos desechables para la hemorragia posparto (HPP), como los taponamientos uterinos con balón y los sistemas de succión, generan importantes residuos médicos, lo que presenta desafíos ambientales y de eliminación, especialmente en entornos de bajos recursos. Sin una infraestructura adecuada para la gestión de residuos, los dispositivos desechados pueden acumularse en vertederos o basureros a cielo abierto, liberando materiales nocivos como plásticos y residuos biopeligrosos al medio ambiente.
- La eliminación inadecuada también aumenta el riesgo de que se deseche o reutilice equipo contaminado en situaciones desesperadas, lo que aumenta el riesgo de infecciones como hepatitis o sepsis entre pacientes y profesionales sanitarios. Esta doble amenaza —la degradación ambiental y los riesgos para la salud pública— subraya la necesidad de soluciones sostenibles de diseño y eliminación en el desarrollo de dispositivos de HPP.
Por ejemplo,
- En marzo de 2022, MDPI destacó que el creciente uso de dispositivos desechables para hemorragias posparto contribuye al creciente volumen de residuos hospitalarios, muchos de los cuales son peligrosos y contribuyen al riesgo de infección y a la contaminación ambiental. La eliminación inadecuada y la creciente dependencia de productos desechables como jeringas y catéteres exigen atención urgente a la gestión sostenible de residuos en la atención materna.
- En diciembre de 2024, según Centurial, si bien los dispositivos desechables para el tratamiento de la HPP mejoran la seguridad y reducen el riesgo de infección, su uso generalizado genera preocupación ambiental. La eliminación inadecuada contribuye a la acumulación de desechos médicos y a la contaminación. Es fundamental equilibrar la necesidad de instrumental estéril de un solo uso con la gestión sostenible de residuos para minimizar el daño ambiental y garantizar una atención materna eficaz.
Alcance del mercado europeo de dispositivos para el tratamiento de hemorragias posparto
El mercado europeo de dispositivos para el tratamiento de hemorragias posparto se clasifica en cinco segmentos notables que se basan en el tipo, la condición, el tipo de paciente, el usuario final y el canal de distribución.
|
Segmentación |
Subsegmentación |
|
Por tipo |
|
|
Por condición |
|
|
Por tipo de paciente |
|
|
Por el usuario final
|
|
|
Por canal de distribución |
|
Análisis regional del mercado europeo de dispositivos para el tratamiento de hemorragias posparto
Alemania es el país líder en el mercado de dispositivos para el tratamiento de hemorragias posparto .
- Alemania cuenta con un sistema de salud consolidado con altos estándares de atención materna. Las políticas proactivas del gobierno y las sustanciales inversiones en salud materna contribuyen a la adopción generalizada de dispositivos avanzados para el tratamiento de la HPP en todo el país.
- El país tiene una población significativa en riesgo de padecer afecciones asociadas con la HPP grave, como embarazos múltiples, fibromas uterinos, anemia y síndrome HELLP. Este elevado riesgo impulsa la demanda de soluciones eficaces para el tratamiento de la HPP, posicionando a Alemania como un mercado líder en Europa.
- Alemania alberga importantes empresas en el sector de dispositivos para el tratamiento de la HPP, como Cook Medical y Utah Medical Products. Estas empresas tienen una sólida presencia en el mercado alemán y ofrecen una gama de productos innovadores, como los taponamientos uterinos con balón, muy demandados por su facilidad de uso y eficacia.
Se proyecta que Alemania registre la CAGR más alta del mercado .
- Alemania tiene una población significativa en riesgo de padecer afecciones asociadas con la HPP grave, como embarazos múltiples, fibromas uterinos, anemia y síndrome HELLP. Este elevado riesgo impulsa la demanda de soluciones eficaces para el tratamiento de la HPP, posicionando a Alemania como un mercado líder en Europa.
- Alemania cuenta con un sistema de salud consolidado con altos estándares de atención materna. Las políticas proactivas del gobierno y las sustanciales inversiones en salud materna contribuyen a la adopción generalizada de dispositivos avanzados para el tratamiento de la HPP en todo el país.
Cuota de mercado de dispositivos para el tratamiento de hemorragias posparto
El panorama competitivo del mercado ofrece detalles por competidor. Se incluye información general de la empresa, sus estados financieros, ingresos generados, potencial de mercado, inversión en investigación y desarrollo, nuevas iniciativas de mercado, presencia en Europa, plantas de producción, capacidad de producción, fortalezas y debilidades de la empresa, lanzamiento de productos, alcance y variedad de productos, y dominio de las aplicaciones. Los datos anteriores se refieren únicamente al enfoque de mercado de las empresas.
Los principales líderes del mercado que operan en el mercado son:
- BD (EE. UU.)
- Grupo de empresas Organon (Países Bajos)
- Laborie (EE. UU.)
- Cooper Companies (EE. UU.)
- Belmont Medical Technologies (EE. UU.)
- Productos médicos de Utah, Inc. (EE. UU.)
- Angiplast Private Limited (India)
- Krishco Medical Products Pvt. Ltd. (India)
- Diseño de la tercera piedra (EE. UU.)
- Advin Health Care (EE. UU.)
- Coagulant Therapeutics Corporation (EE. UU.)
- Grupo Sterimed (EE. UU.)
- RevMedx (EE. UU.)
- Maternova Inc (EE. UU.)
- Sinapi Biomedical (EE. UU.)
Últimos avances en dispositivos para el tratamiento de hemorragias posparto
- En abril de 2025, Organon adquirió los derechos en EE. UU. de TOFIDENCE, un biosimilar de tocilizumab a ACTEMRA, de Biogen. Esto fortalece la cartera de biosimilares de Organon en inmunología, ampliando las opciones de tratamiento para la artritis y la COVID-19. TOFIDENCE, lanzado en mayo de 2024, trata diversas enfermedades inflamatorias e impulsa el crecimiento del negocio de biosimilares de Organon, con un importante potencial de mercado.
- En noviembre de 2023, CooperCompanies adquirió activos selectos de Cook Medical por 300 millones de dólares, lo que reforzó su cartera de salud femenina y cirugía bajo CooperSurgical. La operación incluye productos como el balón Bakri y monitores Doppler. Se prevé que esta adquisición impulse los ingresos y las ganancias en 2024, y refuerza la posición de Cooper en Europa en fertilidad y ginecología.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 MARKET END USER COVERAGE GRID
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTAL ANALYSIS
4.2 PORTERS FIVE FORCES ANALYSIS
4.3 INDUSTRY INSIGHTS
4.3.1 MICRO AND MACROECONOMIC FACTORS
4.3.2 PENETRATION AND GROWTH PROSPECT MAPPING
4.3.3 KEY PRICING STRATEGIES
4.4 COST ANALYSIS BREAKDOWN
4.5 TECHNOLOGY ROADMAP
4.6 VALUE CHAIN ANALYSIS
4.7 OPPORTUNITY MAP ANALYSIS
4.8 HEALTHCARE ECONOMY
4.9 REIMBURSEMENT FRAMEWORK
4.1 TARIFFS AND ITS IMPACT ON THE MARKET
4.10.1 DEFINITION AND IMPORTANCE OF TARIFFS IN THE HEALTHCARE SECTOR
4.10.2 EUROPE VS. REGIONAL TARIFF STRUCTURES
4.10.3 IMPACT OF TARIFFS ON HEALTHCARE COSTS AND ACCESSIBILITY
4.10.4 TARIFF REGULATIONS IN KEY MARKETS
4.10.4.1 MEDICARE/MEDICAID TARIFF POLICIES
4.10.4.2 CMS PRICING MODELS
4.10.4.3 OTHERS
4.10.5 TARIFFS ON MEDICAL DEVICES & EQUIPMENT
4.10.5.1 IMPORT/EXPORT DUTIES ON MEDICAL EQUIPMENT
4.10.5.2 IMPACT ON PRICING AND AVAILABILITY OF HIGH-END MEDICAL TECHNOLOGY
4.10.5.3 CASE STUDIES OF TARIFF CHANGES AFFECTING THE INDUSTRY
4.10.6 COST BURDEN ON HOSPITALS AND HEALTHCARE FACILITIES
4.10.7 TARIFF EXEMPTIONS AND INCENTIVES
4.10.8 DUTY-FREE IMPORTS FOR ESSENTIAL MEDICINES AND VACCINES
4.10.9 IMPACT OF TRADE WARS ON THE HEALTHCARE SUPPLY CHAIN
4.10.10 ROLE OF FREE TRADE AGREEMENTS (FTAS) IN REDUCING TARIFFS
5 REGULATORY FRAMEWORK
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING INCIDENCE OF POSTPARTUM HAEMORRHAGE
6.1.2 ONGOING TECHNOLOGICAL ADVANCEMENTS FOR POSTPARTUM HEMORRHAGE TREATMENTS
6.1.3 RISING BIRTH RATES ASSOCIATED WITH AN INCREASE IN THE NUMBER OF POSTPARTUM HEMORRHAGE
6.1.4 REGULATORY SUPPORT AND APPROVALS ASSOCIATED WITH THE TREATMENT DEVICES
6.2 RESTRAINTS
6.2.1 SIDE EFFECTS ASSOCIATED WITH THE POSTPARTUM HEMORRHAGE TREATMENT
6.2.2 LIMITED RESEARCH AND DEVELOPMENT FOR PPH TREATMENT
6.3 OPPORTUNITIES
6.3.1 TRAINING AND EDUCATIONAL PROGRAMS FOR PROPER USE OF PPH TREATMENT DEVICES
6.3.2 SUPPORT FROM GOVERNMENTAL AND NON-GOVERNMENTAL ORGANIZATIONS IN PPH DEVICE ADOPTION
6.3.3 TELEMEDICINE INTEGRATION TO ENHANCE POSTPARTUM HEMORRHAGE DEVICE USE
6.4 CHALLENGES
6.4.1 ENVIRONMENTAL IMPACT AND DISPOSAL ISSUES OF SINGLE-USE PPH DEVICES
6.4.2 STERILITY CHALLENGES AND INFECTION RISKS IN PPH DEVICES
7 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE
7.1 OVERVIEW
7.2 UTERINE BALLOON TAMPONADE
7.2.1 BAKRI BALLOON
7.2.1.1 BAKRI POSTPARTUM BALLOON
7.2.1.2 BAKRI POSTPARTUM BALLOON WITH RAPID INSTILLATION COMPONENTS
7.2.2 FOLEY CATHETER
7.2.2.1 STANDARD FOLEY CATHETER
7.2.2.2 CONDOM-LOADED FOLEY CATHETER
7.3 UNIJECT PREFILLED INJECTION SYSTEM
7.3.1 OXYTOCIN-BASED INJECTION SYSTEM
7.3.2 CARBETOCIN-BASED INJECTION SYSTEM
7.4 NON-PNEUMATIC ANTI-SHOCK GARMENT
7.4.1 STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT
7.4.1.1 MEDIUM
7.4.1.2 LARGE
7.4.2 MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT
7.4.2.1 MEDIUM
7.4.2.2 LARGE
7.5 VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES
7.5.1 JADA SYSTEM
7.5.2 OTHERS
7.6 OTHERS
7.6.1 COMPRESSION DEVICES
7.6.1.1 B-LYNCH
7.6.1.2 HAYMAN
7.6.1.3 OTHER
7.6.2 UTERINE ARTERY LIGATION PRODUCTS
7.6.3 OTHERS
8 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE
8.1 OVERVIEW
8.2 PRIMARY PPH
8.3 SECONDARY PPH
9 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION
9.1 OVERVIEW
9.2 MAJOR POSTPARTUM HEMORRHAGE (MORE THAN 1000 ML)
9.3 MINOR POSTPARTUM HEMORRHAGE (500-1000 ML)
9.4 MASSIVE POSTPARTUM HEMORRHAGE (2000 ML OR MORE)
9.5 SECONDARY POSTPARTUM HEMORRHAGE
10 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL
10.1 OVERVIEW
10.2 DIRECT TENDER
10.3 RETAIL SALES
10.3.1 OFFLINE SALES
10.3.2 ONLINE SALES
10.4 OTHERS
11 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.2.1 PUBLIC HOSPITALS
11.2.1.1 TIER 2
11.2.1.2 TIER 3
11.2.1.3 TIER 1
11.2.2 PRIVATE HOSPITALS
11.2.2.1 TIER 2
11.2.2.2 TIER 3
11.2.2.3 TIER 1
11.3 MATERNITY CENTERS
11.4 SPECIALTY CLINICS
11.5 HOME CARE SETTINGS
11.6 OTHERS
12 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION
12.1 EUROPE
12.1.1 GERMANY
12.1.2 FRANCE
12.1.3 U.K.
12.1.4 ITALY
12.1.5 SPAIN
12.1.6 RUSSIA
12.1.7 TURKEY
12.1.8 NETHERLAND
12.1.9 POLAND
12.1.10 BELGIUM
12.1.11 SWITZERLAND
12.1.12 SWEDEN
12.1.13 DENMARK
12.1.14 NORWAY
12.1.15 FINLAND
12.1.16 REST OF EUROPE
13 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: GLOBAL
14 SWOT ANALYSIS
15 COMPANY PROFILES
15.1 BD
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 SOLUTION PORTFOLIO
15.1.5 RECENT NEWS
15.2 ORGANON GROUP OF COMPANIES
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENTS/NEWS
15.3 LABORIE
15.3.1 COMPANY SNAPSHOT
15.3.2 COMPANY SHARE ANALYSIS
15.3.3 PRODUCT PORTFOLIO
15.3.4 RECENT DEVELOPMENT
15.4 COOPERCOMPANIES
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENT
15.5 BELMONT MEDICAL TECHNOLOGIES
15.5.1 COMPANY SNAPSHOT
15.5.2 COMPANY SHARE ANALYSIS
15.5.3 PRODUCT PORTFOLIO
15.5.4 RECENT DEVELOPMENT
15.6 ADVIN HEALTH CARE
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENTS
15.7 ANGIPLAST PRIVATE LIMITED
15.7.1 COMPANY SNAPSHOT
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENT
15.8 COAGULANT THERAPEUTICS
15.8.1 COMPANY SNAPSHOT
15.8.2 PIPELINE PORTFOLIO
15.8.3 RECENT DEVELOPMENT
15.9 KRISHCO MEDICAL PRODUCTS PVT. LTD
15.9.1 COMPANY SNAPSHOT
15.9.2 PRODUCT PORTFOLIO
15.9.3 RECENT DEVELOPMENT
15.1 MATERNOVA INC.
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENT
15.11 REVMEDX
15.11.1 COMPANY SNAPSHOT
15.11.2 PRODUCT PORTFOLIO
15.11.3 RECENT DEVELOPMENT
15.12 3RD STONE DESIGN
15.12.1 COMPANY SNAPSHOT
15.12.2 PRODUCT PORTFOLIO
15.12.3 RECENT DEVELOPMENTS
15.13 STERIMED GROUP
15.13.1 COMPANY SNAPSHOT
15.13.2 PRODUCT PORTFOLIO
15.13.3 RECENT DEVELOPMENTS
15.14 UTAH MEDICAL PRODUCTS, INC.
15.14.1 COMPANY SNAPSHOT
15.14.2 REVENUE ANALYSIS
15.14.3 PRODUCT PORTFOLIO
15.14.4 RECENT DEVELOPMENTS
15.15 SINAPI BIOMEDICAL
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENTS
16 QUESTIONNAIRE
Lista de Tablas
TABLE 1 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 2 EUROPE UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 3 EUROPE UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 4 EUROPE BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 5 EUROPE FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 6 EUROPE UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 7 EUROPE UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 8 EUROPE NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 9 EUROPE NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 10 EUROPE STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 11 EUROPE MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 12 EUROPE VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 13 EUROPE VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 14 EUROPE OTHERS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 15 EUROPE OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 16 EUROPE COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 17 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 18 EUROPE PRIMARY PPH IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 19 EUROPE SECONDARY PPH IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 20 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 21 EUROPE MAJOR POSTPARTUM HEMORRHAGE (MORE THAN 1000 ML) IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 22 EUROPE MINOR POSTPARTUM HEMORRHAGE (500-1000 ML) IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 23 EUROPE MASSIVE POSTPARTUM HEMORRHAGE (2000 ML OR MORE) IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 24 EUROPE SECONDARY POSTPARTUM HEMORRHAGE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 25 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 26 EUROPE DIRECT TENDER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 27 EUROPE RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 28 EUROPE RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 29 EUROPE OTHERS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 30 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 31 EUROPE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 32 EUROPE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 33 EUROPE PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 34 EUROPE PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 35 EUROPE MATERNITY CENTERS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 36 EUROPE SPECIALTY CLINICS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 37 EUROPE HOME CARE SETTINGS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 38 EUROPE OTHERS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 39 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 40 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 41 EUROPE UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 42 EUROPE BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 43 EUROPE FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 44 EUROPE UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 45 EUROPE NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 46 EUROPE STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 47 EUROPE MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 48 EUROPE VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 49 EUROPE OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 50 EUROPE COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 51 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 52 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 53 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 54 EUROPE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 55 EUROPE PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 56 EUROPE PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 57 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 58 EUROPE RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 59 GERMANY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 60 GERMANY UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 61 GERMANY BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 62 GERMANY FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 63 GERMANY UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 64 GERMANY NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 65 GERMANY STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 66 GERMANY MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 67 GERMANY VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 68 GERMANY OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 69 GERMANY COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 70 GERMANY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 71 GERMANY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 72 GERMANY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 73 GERMANY HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 74 GERMANY PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 75 GERMANY PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 76 GERMANY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 77 GERMANY RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 78 FRANCE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 79 FRANCE UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 80 FRANCE BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 81 FRANCE FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 82 FRANCE UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 83 FRANCE NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 84 FRANCE STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 85 FRANCE MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 86 FRANCE VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 87 FRANCE OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 88 FRANCE COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 89 FRANCE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 90 FRANCE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 91 FRANCE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 92 FRANCE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 93 FRANCE PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 94 FRANCE PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 95 FRANCE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 96 FRANCE RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 97 U.K. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 98 U.K. UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 99 U.K. BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 100 U.K. FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 101 U.K. UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 102 U.K. NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 103 U.K. STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 104 U.K. MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 105 U.K. VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 106 U.K. OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 107 U.K. COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 108 U.K. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 109 U.K. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 110 U.K. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 111 U.K. HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 112 U.K. PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 113 U.K. PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 114 U.K. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 115 U.K. RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 116 ITALY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 117 ITALY UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 118 ITALY BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 119 ITALY FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 120 ITALY UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 121 ITALY NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 122 ITALY STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 123 ITALY MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 124 ITALY VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 125 ITALY OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 126 ITALY COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 127 ITALY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 128 ITALY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 129 ITALY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 130 ITALY HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 131 ITALY PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 132 ITALY PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 133 ITALY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 134 ITALY RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 135 SPAIN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 136 SPAIN UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 137 SPAIN BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 138 SPAIN FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 139 SPAIN UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 140 SPAIN NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 141 SPAIN STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 142 SPAIN MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 143 SPAIN VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 144 SPAIN OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 145 SPAIN COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 146 SPAIN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 147 SPAIN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 148 SPAIN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 149 SPAIN HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 150 SPAIN PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 151 SPAIN PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 152 SPAIN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 153 SPAIN RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 154 RUSSIA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 155 RUSSIA UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 156 RUSSIA BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 157 RUSSIA FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 158 RUSSIA UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 159 RUSSIA NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 160 RUSSIA STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 161 RUSSIA MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 162 RUSSIA VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 163 RUSSIA OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 164 RUSSIA COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 165 RUSSIA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 166 RUSSIA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 167 RUSSIA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 168 RUSSIA HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 169 RUSSIA PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 170 RUSSIA PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 171 RUSSIA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 172 RUSSIA RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 173 TURKEY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 174 TURKEY UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 175 TURKEY BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 176 TURKEY FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 177 TURKEY UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 178 TURKEY NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 179 TURKEY STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 180 TURKEY MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 181 TURKEY VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 182 TURKEY OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 183 TURKEY COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 184 TURKEY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 185 TURKEY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 186 TURKEY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 187 TURKEY HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 188 TURKEY PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 189 TURKEY PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 190 TURKEY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 191 TURKEY RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 192 NETHERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 193 NETHERLAND UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 194 NETHERLAND BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 195 NETHERLAND FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 196 NETHERLAND UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 197 NETHERLAND NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 198 NETHERLAND STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 199 NETHERLAND MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 200 NETHERLAND VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 201 NETHERLAND OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 202 NETHERLAND COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 203 NETHERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 204 NETHERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 205 NETHERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 206 NETHERLAND HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 207 NETHERLAND PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 208 NETHERLAND PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 209 NETHERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 210 NETHERLAND RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 211 POLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 212 POLAND UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 213 POLAND BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 214 POLAND FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 215 POLAND UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 216 POLAND NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 217 POLAND STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 218 POLAND MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 219 POLAND VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 220 POLAND OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 221 POLAND COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 222 POLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 223 POLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 224 POLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 225 POLAND HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 226 POLAND PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 227 POLAND PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 228 POLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 229 POLAND RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 230 BELGIUM POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 231 BELGIUM UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 232 BELGIUM BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 233 BELGIUM FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 234 BELGIUM UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 235 BELGIUM NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 236 BELGIUM STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 237 BELGIUM MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 238 BELGIUM VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 239 BELGIUM OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 240 BELGIUM COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 241 BELGIUM POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 242 BELGIUM POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 243 BELGIUM POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 244 BELGIUM HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 245 BELGIUM PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 246 BELGIUM PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 247 BELGIUM POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 248 BELGIUM RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 249 SWITZERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 250 SWITZERLAND UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 251 SWITZERLAND BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 252 SWITZERLAND FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 253 SWITZERLAND UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 254 SWITZERLAND NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 255 SWITZERLAND STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 256 SWITZERLAND MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 257 SWITZERLAND VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 258 SWITZERLAND OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 259 SWITZERLAND COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 260 SWITZERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 261 SWITZERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 262 SWITZERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 263 SWITZERLAND HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 264 SWITZERLAND PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 265 SWITZERLAND PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 266 SWITZERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 267 SWITZERLAND RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 268 SWEDEN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 269 SWEDEN UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 270 SWEDEN BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 271 SWEDEN FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 272 SWEDEN UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 273 SWEDEN NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 274 SWEDEN STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 275 SWEDEN MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 276 SWEDEN VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 277 SWEDEN OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 278 SWEDEN COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 279 SWEDEN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 280 SWEDEN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 281 SWEDEN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 282 SWEDEN HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 283 SWEDEN PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 284 SWEDEN PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 285 SWEDEN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 286 SWEDEN RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 287 DENMARK POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 288 DENMARK UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 289 DENMARK BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 290 DENMARK FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 291 DENMARK UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 292 DENMARK NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 293 DENMARK STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 294 DENMARK MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 295 DENMARK VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 296 DENMARK OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 297 DENMARK COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 298 DENMARK POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 299 DENMARK POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 300 DENMARK POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 301 DENMARK HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 302 DENMARK PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 303 DENMARK PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 304 DENMARK POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 305 DENMARK RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 306 NORWAY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 307 NORWAY UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 308 NORWAY BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 309 NORWAY FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 310 NORWAY UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 311 NORWAY NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 312 NORWAY STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 313 NORWAY MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 314 NORWAY VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 315 NORWAY OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 316 NORWAY COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 317 NORWAY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 318 NORWAY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 319 NORWAY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 320 NORWAY HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 321 NORWAY PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 322 NORWAY PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 323 NORWAY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 324 NORWAY RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 325 FINLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 326 FINLAND UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 327 FINLAND BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 328 FINLAND FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 329 FINLAND UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 330 FINLAND NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 331 FINLAND STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 332 FINLAND MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 333 FINLAND VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 334 FINLAND OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 335 FINLAND COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 336 FINLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 337 FINLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 338 FINLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 339 FINLAND HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 340 FINLAND PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 341 FINLAND PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 342 FINLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 343 FINLAND RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 344 REST OF EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
Lista de figuras
FIGURE 1 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: SEGMENTATION
FIGURE 2 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: DROC ANALYSIS
FIGURE 4 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: VENDOR SHARE ANALYSIS
FIGURE 9 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: MARKET END USER COVERAGE GRID
FIGURE 10 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: SEGMENTATION
FIGURE 11 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: EXECUTIVE SUMMARY
FIGURE 12 STRATEGIC DECISIONS
FIGURE 13 RISING INCIDENCE OF POSTPARTUM HAEMORRHAGE IS EXPECTED TO DRIVE THE EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 14 UTERINE BALLOON TAMPONADE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET IN THE FORECAST PERIOD OF 2025 & 2032
FIGURE 15 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE (2024)
FIGURE 16 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET
FIGURE 17 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY TYPE, 2024
FIGURE 18 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY TYPE, 2025-2032 (USD THOUSAND)
FIGURE 19 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY TYPE, CAGR (2025-2032)
FIGURE 20 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY TYPE, LIFELINE CURVE
FIGURE 21 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY PATIENT TYPE, 2024
FIGURE 22 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY PATIENT TYPE, 2025-2032 (USD THOUSAND)
FIGURE 23 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY PATIENT TYPE, CAGR (2025-2032)
FIGURE 24 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY PATIENT TYPE, LIFELINE CURVE
FIGURE 25 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY CONDITION, 2024
FIGURE 26 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY CONDITION, 2025-2032 (USD THOUSAND)
FIGURE 27 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY CONDITION, CAGR (2025-2032)
FIGURE 28 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY CONDITION, LIFELINE CURVE
FIGURE 29 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 30 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY DISTRIBUTION CHANNEL, 2025-2032 (USD THOUSAND)
FIGURE 31 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025-2032)
FIGURE 32 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 33 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY END USER, 2024
FIGURE 34 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY END USER, 2025-2032 (USD THOUSAND)
FIGURE 35 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY END USER, CAGR (2025-2032)
FIGURE 36 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY END USER, LIFELINE CURVE
FIGURE 37 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: SNAPSHOT (2024)
FIGURE 38 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: COMPANY SHARE 2024 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.