Europe Sarcopenia Treatment Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
789.43 Million
USD
1,131.28 Million
2024
2032
USD
789.43 Million
USD
1,131.28 Million
2024
2032
| 2025 –2032 | |
| USD 789.43 Million | |
| USD 1,131.28 Million | |
|
|
|
|
Segmentación del mercado europeo de tratamiento de la sarcopenia por tipo de tratamiento (medicamentos, suplementos vitamínicos/dietéticos, etc.), tipo (sarcopenia primaria y secundaria), estadios (presarcopenia, sarcopenia y sarcopenia grave), vía de administración (oral, inyectable, etc.), género (masculino y femenino), usuario final (hospitales, clínicas especializadas, atención médica domiciliaria, etc.), canal de distribución (licitación directa, venta minorista, etc.): tendencias del sector y pronóstico hasta 2032.
Tamaño del mercado europeo de tratamiento de la sarcopenia
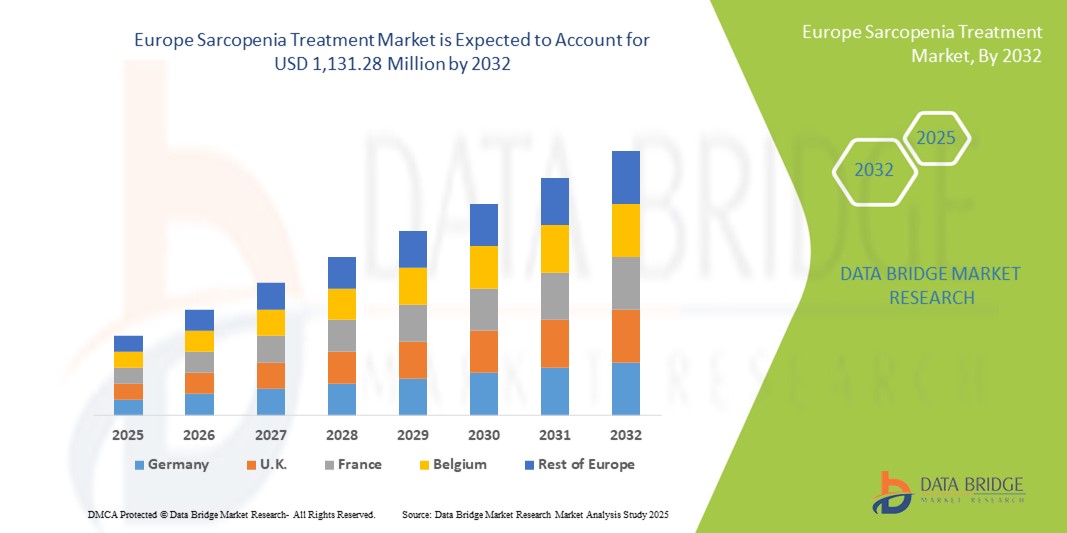
- El tamaño del mercado europeo de tratamiento de la sarcopenia se valoró en USD 789,43 millones en 2024 y se espera que alcance los USD 1.131,28 millones para 2032 , con una CAGR del 4,6 % durante el período de pronóstico.
- El crecimiento del mercado se ve impulsado en gran medida por la creciente prevalencia de la desnutrición, las deficiencias vitamínicas y la pérdida muscular relacionada con la edad, junto con una creciente conciencia sobre el impacto de la sarcopenia en la salud y la calidad de vida.
- Además, la creciente demanda de opciones de tratamiento eficaces, como suplementos nutricionales, fisioterapia y medicamentos, está consolidando los tratamientos para la sarcopenia como esenciales para las poblaciones envejecidas. Estos factores convergentes están acelerando la adopción de soluciones terapéuticas, impulsando así significativamente el crecimiento de la industria.
Análisis del mercado europeo de tratamiento de la sarcopenia
- Los tratamientos para la sarcopenia, que abarcan suplementos nutricionales, fisioterapia e intervenciones farmacológicas, son cada vez más vitales para controlar la pérdida muscular relacionada con la edad y mantener la independencia funcional entre la población de edad avanzada en Europa debido a su eficacia para mejorar la masa muscular, la fuerza y la calidad de vida en general.
- La creciente demanda de tratamientos para la sarcopenia se debe principalmente al envejecimiento de la población europea, la creciente conciencia sobre los impactos de la sarcopenia en la salud y el creciente énfasis en la atención preventiva y los programas de envejecimiento saludable.
- Alemania dominó el mercado de tratamiento de la sarcopenia con la mayor participación en los ingresos del 23,9 % en 2024, caracterizado por una infraestructura de atención médica avanzada, un alto gasto en atención médica e iniciativas gubernamentales proactivas que promueven el cuidado de los ancianos, con una amplia adopción de programas de suplementación nutricional y fisioterapia.
- Se espera que Italia sea el país de más rápido crecimiento en el mercado de tratamiento de la sarcopenia durante el período de pronóstico debido al aumento de las inversiones en atención médica, el crecimiento de la población geriátrica y la creciente conciencia de la pérdida muscular relacionada con la edad.
- El segmento de suplementos dietéticos dominó el mercado de tratamiento de la sarcopenia con una participación de mercado del 39,2 % en 2024, impulsado por el papel bien establecido de las proteínas, la vitamina D y el calcio en el mantenimiento y la recuperación muscular entre los adultos mayores.
Alcance del informe y segmentación del mercado europeo de tratamiento de la sarcopenia
|
Atributos |
Perspectivas clave del mercado europeo del tratamiento de la sarcopenia |
|
Segmentos cubiertos |
|
|
Países cubiertos |
Europa
|
|
Actores clave del mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de información de datos de valor añadido |
Además de los conocimientos sobre escenarios de mercado como valor de mercado, tasa de crecimiento, segmentación, cobertura geográfica y actores principales, los informes de mercado seleccionados por Data Bridge Market Research también incluyen análisis de expertos en profundidad, análisis de precios, análisis de participación de marca, encuesta de consumidores, análisis demográfico, análisis de la cadena de suministro, análisis de la cadena de valor, descripción general de materias primas/consumibles, criterios de selección de proveedores, análisis PESTLE, análisis de Porter y marco regulatorio. |
Tendencias del mercado europeo de tratamiento de la sarcopenia
Integración de la Salud Digital y la Telemedicina
- Una tendencia significativa y en aceleración en el mercado europeo de tratamiento de la sarcopenia es la integración de soluciones de salud digital y plataformas de telemedicina, que permiten a los pacientes de edad avanzada controlar la salud muscular, hacer un seguimiento de la nutrición y recibir orientación remota de profesionales de la salud.
- Por ejemplo, el programa de telesalud NutriTrack permite a los pacientes registrar la ingesta dietética y los ejercicios de fortalecimiento muscular, mientras que los fisioterapeutas brindan recomendaciones en tiempo real para los ajustes de la terapia.
- La integración de la salud digital permite el seguimiento continuo del progreso del paciente, ofreciendo planes de tratamiento personalizados y recordatorios de suplementación y ejercicios, mejorando la adherencia y los resultados.
- La combinación perfecta de sensores portátiles , aplicaciones móviles y teleconsultas facilita la gestión centralizada del tratamiento de la sarcopenia, lo que permite a los médicos coordinar intervenciones nutricionales, terapéuticas y farmacológicas de manera eficiente.
- Esta tendencia hacia la atención basada en tecnología está cambiando las expectativas para el manejo de la sarcopenia, con empresas como PhysioPlus desarrollando plataformas que integran datos de pacientes, supervisión remota de ejercicios y entrenamiento digital.
- La demanda de tratamientos para la sarcopenia con apoyo de salud digital está creciendo rápidamente en hospitales, clínicas ambulatorias y atención domiciliaria, a medida que los pacientes priorizan cada vez más la conveniencia, el monitoreo y las intervenciones personalizadas.
Dinámica del mercado europeo del tratamiento de la sarcopenia
Conductor
Aumento de la población geriátrica y concienciación sobre la pérdida muscular relacionada con la edad
- El creciente envejecimiento de la población en Europa, combinado con una mayor conciencia del impacto de la sarcopenia en la movilidad y la calidad de vida, es un factor importante para la mayor demanda de tratamientos efectivos.
- Por ejemplo, en 2024, Alemania lanzó el “Programa de Envejecimiento Saludable”, que promueve la suplementación nutricional y las intervenciones de ejercicio en centros de atención para personas mayores para mitigar la prevalencia de la sarcopenia.
- A medida que los adultos mayores experimentan un mayor riesgo de caídas, fragilidad y deterioro funcional, los proveedores de atención médica y los cuidadores buscan cada vez más intervenciones que mejoren la masa muscular y la fuerza.
- El creciente énfasis en la atención sanitaria preventiva, incluido el diagnóstico temprano y el tratamiento oportuno de la sarcopenia, está acelerando la adopción de suplementos nutricionales, fisioterapia y opciones farmacológicas.
- Las campañas de salud pública y los programas geriátricos están promoviendo la concienciación sobre la sarcopenia, impulsando tanto la demanda de los pacientes como la participación de los proveedores de atención médica en la entrega de soluciones de tratamiento específicas.
Restricción/Desafío
Accesibilidad limitada y altos costos de tratamiento
- Las preocupaciones en torno a la asequibilidad y la accesibilidad de los tratamientos para la sarcopenia plantean un desafío importante para una adopción más amplia en el mercado de toda Europa.
- Por ejemplo, a pesar de la evidencia que apoya la suplementación de proteínas y la fisioterapia , algunos pacientes de edad avanzada en Europa del Este tienen acceso limitado a estas intervenciones debido a los altos costos o la infraestructura de atención médica insuficiente.
- La variabilidad en las políticas de reembolso de atención médica entre países puede restringir el acceso de los pacientes a suplementos nutricionales, medicamentos o sesiones de fisioterapia especializada.
- Si bien la eficacia del tratamiento está bien documentada, la falta de pautas estandarizadas y la conciencia limitada entre los cuidadores pueden reducir la adherencia y la adopción de las terapias.
- Los ensayos clínicos para el tratamiento de la sarcopenia pueden utilizar diferentes criterios diagnósticos, lo que dificulta la comparación de los resultados de diversos estudios. Esta falta de estandarización puede dificultar el desarrollo y la aprobación de nuevos tratamientos por parte de las agencias reguladoras.
- Superar estos desafíos a través de programas apoyados por el gobierno, opciones de terapia asequibles e iniciativas educativas para pacientes y cuidadores será vital para el crecimiento sostenido del mercado.
Alcance del mercado europeo de tratamiento de la sarcopenia
El mercado está segmentado según el tipo de tratamiento, tipo de sarcopenia, etapas, vía de administración, género, usuario final y canal de distribución.
- Por tipo de tratamiento
Según el tipo de tratamiento, el mercado de tratamiento de la sarcopenia se segmenta en medicamentos, suplementos vitamínicos/dietéticos y otros. El segmento de suplementos vitamínicos/dietéticos dominó el mercado con la mayor participación en los ingresos, un 39,2 % en 2024, impulsado por el uso generalizado de suplementos de proteínas, vitamina D y calcio entre la población de edad avanzada para prevenir la pérdida muscular. Los suplementos son preferidos por su seguridad, facilidad de administración y capacidad para mantener la masa y la función muscular. La creciente concienciación sobre las intervenciones nutricionales y los programas gubernamentales de apoyo impulsa aún más su adopción. Los pacientes suelen recibir suplementos como primera opción terapéutica, tanto en la sarcopenia primaria como en la secundaria. El mercado registra una demanda constante gracias a la disponibilidad de alimentos fortificados y fórmulas especializadas ricas en proteínas. Los profesionales de la salud recomiendan los suplementos como parte de la atención preventiva y terapéutica para las personas mayores.
Se prevé que el segmento de medicamentos experimente la tasa de crecimiento más rápida, del 11,8 %, entre 2025 y 2032, impulsada por nuevos tratamientos farmacológicos dirigidos a la atrofia muscular, terapias hormonales e inhibidores de la miostatina. Cada vez se recetan más medicamentos a pacientes con sarcopenia grave o secundaria a enfermedades crónicas. El aumento de la inversión en I+D y las aprobaciones regulatorias en los países europeos está acelerando su adopción en el mercado. Los medicamentos ofrecen beneficios específicos y clínicamente validados para mejorar la masa y la fuerza muscular. Las campañas de concienciación promueven su uso junto con suplementos y fisioterapia. Las terapias avanzadas ofrecen potencial para estrategias de tratamiento combinado, lo que impulsa aún más el crecimiento del segmento.
- Por tipo
Según el tipo, el mercado de tratamiento de la sarcopenia se segmenta en sarcopenia primaria y sarcopenia secundaria. El segmento de sarcopenia primaria dominó el mercado con la mayor participación en los ingresos, con un 55,3 % en 2024, impulsado por la pérdida muscular relacionada con la edad que afecta a la población de edad avanzada en toda Europa. Los programas preventivos y de intervención temprana dirigidos al deterioro relacionado con la edad son comunes en países como Alemania y Francia. La sarcopenia primaria suele controlarse mediante intervenciones en el estilo de vida, suplementos nutricionales y fisioterapia. Las campañas de concienciación y los programas de atención geriátrica apoyan su amplia adopción. La prevalencia de la sarcopenia primaria en poblaciones mayores garantiza una demanda constante. Los hospitales y las clínicas integran medidas preventivas, manteniendo así un dominio constante del mercado.
Se prevé que el segmento de la sarcopenia secundaria experimente la tasa de crecimiento más rápida, del 12,3 %, entre 2025 y 2032, impulsada por la creciente incidencia de la sarcopenia debida a enfermedades crónicas como la diabetes, la EPOC y el cáncer. El tratamiento suele requerir una combinación de medicamentos, suplementos y fisioterapia supervisada. La creciente investigación sobre la sarcopenia asociada a enfermedades respalda el desarrollo de terapias dirigidas. La creciente prevalencia de enfermedades crónicas y relacionadas con el estilo de vida en Europa impulsa su adopción. Los programas clínicos incluyen cada vez más intervenciones para la sarcopenia secundaria. La concienciación entre profesionales sanitarios y pacientes contribuye a acelerar la adopción de las terapias.
- Por etapas
Según las etapas, el mercado del tratamiento de la sarcopenia se segmenta en presarcopenia, sarcopenia y sarcopenia grave. La etapa de sarcopenia dominó el mercado con la mayor participación en los ingresos, con un 47,8 % en 2024, ya que los pacientes suelen ser diagnosticados en etapas tempranas a moderadas. Las intervenciones que incluyen nutrición, ejercicio y medicación son más eficaces en esta etapa. Las pruebas de detección periódicas y los programas geriátricos apoyan la adopción temprana del tratamiento. Los hospitales y las clínicas especializadas priorizan las intervenciones oportunas para prevenir la progresión. Las campañas de concienciación y las estrategias de gestión proactiva contribuyen a su dominio del mercado. La adopción se ve impulsada por la mejora de los resultados de los pacientes y la reducción del riesgo de deterioro de la movilidad.
Se prevé que el segmento de la sarcopenia grave experimente la tasa de crecimiento más rápida, del 13,1 %, entre 2025 y 2032, impulsada por un número creciente de pacientes mayores con pérdida muscular avanzada. En esta etapa, se requieren terapias combinadas intensivas que incluyan medicamentos, suplementos y fisioterapia. Los profesionales sanitarios se centran en mejorar la movilidad, reducir el riesgo de caídas y mejorar la calidad de vida. La creciente prevalencia de la sarcopenia grave en la población de edad avanzada impulsa la demanda de intervenciones eficaces. Se están implementando programas de manejo de la etapa avanzada en hospitales y clínicas. El aumento de la población geriátrica garantiza un crecimiento sostenido del mercado en este segmento.
- Por vía de administración
Según la vía de administración, el mercado del tratamiento de la sarcopenia se segmenta en oral, inyectable y otros. El segmento oral dominó el mercado con la mayor participación en los ingresos, con un 53,7%, en 2024, gracias a la facilidad de administración oral de suplementos dietéticos y medicamentos. Los pacientes prefieren los tratamientos orales por su comodidad, cumplimiento terapéutico y seguridad. Los suplementos y medicamentos están ampliamente disponibles sin receta y se recomiendan tanto para la sarcopenia primaria como para la secundaria. Los productos orales incluyen alimentos fortificados, cápsulas y polvos diseñados para personas mayores. Su adopción se ve respaldada por programas de atención médica domiciliaria y recomendaciones clínicas. El crecimiento del mercado se ve impulsado aún más por las campañas de concienciación pública que promueven la suplementación oral.
Se prevé que el segmento de inyectables experimente la tasa de crecimiento más rápida, del 14,2 %, entre 2025 y 2032, impulsada por el desarrollo de terapias inyectables dirigidas a la sarcopenia grave y tratamientos anabólicos. Los medicamentos inyectables proporcionan una dosificación precisa y una eficacia más rápida en casos avanzados. Los hospitales y las clínicas especializadas están adoptando cada vez más opciones inyectables para mejorar los resultados de los pacientes. Las terapias avanzadas permiten la recuperación muscular dirigida en pacientes con sarcopenia crónica o grave. Los ensayos clínicos y las aprobaciones en los mercados europeos están impulsando su adopción. La creciente concienciación sobre la eficacia de los tratamientos entre los profesionales sanitarios y los pacientes impulsa el crecimiento del segmento.
- Por género
En función del género, el mercado del tratamiento de la sarcopenia se segmenta en hombres y mujeres. El segmento femenino dominó el mercado con la mayor participación en los ingresos, con un 51,5 % en 2024, debido a la mayor prevalencia de sarcopenia en mujeres mayores, especialmente en la posmenopausia, debido a los cambios hormonales que afectan la masa muscular. Se recomiendan comúnmente intervenciones nutricionales, fisioterapia y medicamentos para las mujeres. Las iniciativas de salud pública centradas en la salud femenina fomentan aún más su adopción. Las campañas de concienciación se centran en la detección y el tratamiento tempranos en mujeres. Los profesionales sanitarios priorizan las intervenciones personalizadas para mujeres mayores. La combinación de atención preventiva y tratamientos terapéuticos garantiza una demanda sostenida.
Se prevé que el segmento masculino experimente la tasa de crecimiento más rápida, del 10,9 %, entre 2025 y 2032, impulsada por la creciente concienciación sobre la sarcopenia en hombres mayores y la mayor participación en programas de atención preventiva. Las intervenciones en el estilo de vida, la suplementación y las terapias clínicas están ganando terreno entre los hombres. Los programas gubernamentales y privados promueven la detección temprana en hombres. El mayor enfoque en la movilidad, la fuerza y la independencia funcional impulsa la adopción. La investigación clínica que destaca la sarcopenia en hombres respalda el crecimiento. El aumento de la población masculina geriátrica garantiza la expansión del mercado en este segmento.
- Por el usuario final
Según el usuario final, el mercado del tratamiento de la sarcopenia se segmenta en hospitales, clínicas especializadas, atención médica domiciliaria y otros. Los hospitales dominaron el mercado con la mayor participación en los ingresos, con un 46,2 % en 2024, ofreciendo un tratamiento integral para la sarcopenia, que incluye diagnóstico, suplementación, medicamentos y programas de fisioterapia. Los hospitales han establecido programas de atención geriátrica, lo que facilita la adopción del tratamiento. La detección temprana y el seguimiento continuo en los hospitales garantizan resultados óptimos para los pacientes. Los hospitales suelen colaborar con la atención médica domiciliaria y las clínicas para ampliar la atención. Los hospitales públicos y privados promueven activamente iniciativas de atención preventiva. El enfoque centralizado del tratamiento en los hospitales garantiza una demanda constante del mercado.
Se prevé que el segmento de atención médica domiciliaria experimente la tasa de crecimiento más rápida, del 15,3 %, entre 2025 y 2032, impulsada por la creciente demanda de atención domiciliaria para personas mayores, así como por intervenciones nutricionales y de fisioterapia domiciliarias. Los programas de monitorización remota y telemedicina permiten una gestión eficaz fuera del ámbito hospitalario. Los programas de atención domiciliaria personalizada mejoran el cumplimiento y los resultados del tratamiento. Este crecimiento se sustenta en una mayor concienciación sobre la comodidad y la independencia entre los pacientes mayores. Los proveedores de atención médica domiciliaria integran herramientas digitales para la monitorización en tiempo real. El segmento se beneficia de las políticas sanitarias que promueven estrategias de envejecimiento en el hogar.
- Por canal de distribución
Según el canal de distribución, el mercado del tratamiento de la sarcopenia se segmenta en licitación directa, venta minorista y otros. El segmento de venta minorista dominó el mercado con la mayor participación en los ingresos, con un 49,6 % en 2024, impulsado por la amplia disponibilidad de suplementos y medicamentos en farmacias, supermercados y plataformas de comercio electrónico. La fácil accesibilidad, la disponibilidad de medicamentos de venta libre y la conveniencia respaldan este dominio del mercado. Los consumidores prefieren el comercio minorista para compras frecuentes y acceso inmediato. La expansión del comercio minorista en zonas urbanas y semiurbanas contribuye al crecimiento de los ingresos. Las campañas de marketing de las marcas dirigidas a cuidadores y personas mayores aumentan el conocimiento del mercado. El segmento se beneficia de redes de distribución consolidadas y de la visibilidad de la marca.
Se prevé que el segmento de licitación directa registre la tasa de crecimiento más rápida, del 12,7 %, entre 2025 y 2032, impulsada por el aumento de la adquisición de productos para el tratamiento de la sarcopenia por parte de hospitales, clínicas especializadas y programas gubernamentales para el cuidado de personas mayores. Los pedidos por licitación a granel garantizan un suministro constante para uso institucional. La creciente concienciación sobre los programas de atención preventiva y para el envejecimiento impulsa la distribución por licitación. Las iniciativas gubernamentales de salud dependen cada vez más del suministro directo de suplementos y medicamentos. La adopción institucional garantiza un mayor volumen de ventas e ingresos sostenidos. Las alianzas con fabricantes y distribuidores aceleran el crecimiento del mercado en este canal.
Análisis regional del mercado europeo de tratamiento de la sarcopenia
- Alemania dominó el mercado de tratamiento de la sarcopenia con la mayor participación en los ingresos del 23,9 % en 2024, caracterizado por una infraestructura de atención médica avanzada, un alto gasto en atención médica e iniciativas gubernamentales proactivas que promueven el cuidado de los ancianos, con una amplia adopción de programas de suplementación nutricional y fisioterapia.
- Los pacientes y los proveedores de atención médica del país adoptan cada vez más suplementos nutricionales, fisioterapia y medicamentos para la intervención temprana y el tratamiento de la sarcopenia.
- La adopción generalizada está respaldada además por programas gubernamentales proactivos, campañas de salud pública y sistemas de atención geriátrica bien establecidos, lo que hace que los tratamientos de sarcopenia sean un componente fundamental de la atención médica para personas mayores.
Análisis del mercado alemán de tratamiento de la sarcopenia
El mercado alemán de tratamiento de la sarcopenia registró la mayor participación en ingresos de Europa en 2024, impulsado por centros de salud avanzados y programas de atención geriátrica consolidados. Los profesionales sanitarios priorizan la detección temprana y el manejo de la sarcopenia mediante suplementos dietéticos, fisioterapia y medicamentos. Las campañas de salud pública y las iniciativas gubernamentales de atención a personas mayores promueven el conocimiento y la adopción de tratamientos. El fuerte enfoque de Alemania en la atención preventiva, sumado al elevado gasto sanitario, fomenta la integración de enfoques multimodales para el manejo de la sarcopenia. El mercado está experimentando un crecimiento en hospitales, clínicas y atención domiciliaria, lo que refleja una amplia aceptación de las soluciones terapéuticas. Los pacientes se benefician de opciones terapéuticas accesibles y de la integración tecnológica en el seguimiento y la atención.
Análisis del mercado del tratamiento de la sarcopenia en Francia
Se proyecta que el mercado francés de tratamiento de la sarcopenia se expandirá a una tasa de crecimiento anual compuesta (TCAC) significativa durante el período de pronóstico, impulsado principalmente por el envejecimiento de la población del país y la creciente concienciación sobre las prácticas de envejecimiento saludable. Los profesionales sanitarios franceses recomiendan cada vez más suplementos nutricionales y fisioterapia para prevenir y controlar la sarcopenia. Las iniciativas gubernamentales y los programas de salud preventiva dirigidos a las personas mayores impulsan aún más el crecimiento del mercado. La integración de la telemedicina y las plataformas de salud digital mejora la adherencia del paciente y el seguimiento del tratamiento. Los segmentos de atención médica residencial, ambulatoria y domiciliaria están experimentando una mayor adopción. La importancia de la calidad de vida y la independencia funcional entre las personas mayores es un factor clave para el mercado en Francia.
Análisis del mercado italiano del tratamiento de la sarcopenia
Se espera que el mercado italiano de tratamiento de la sarcopenia crezca a una tasa de crecimiento anual compuesta (TCAC) notable, impulsado por el aumento de la población geriátrica y la creciente concienciación sobre los riesgos para la salud relacionados con la pérdida muscular. Los profesionales sanitarios italianos promueven la intervención temprana mediante suplementos, programas de ejercicio y medicamentos. Las iniciativas gubernamentales y las campañas de salud preventiva impulsan la adopción, especialmente en entornos de atención médica comunitarios y domiciliarios. Los pacientes participan cada vez más en programas de autocuidado, respaldados por la salud digital y la telemonitorización. Los hospitales y las clínicas especializadas continúan ampliando su oferta de servicios para abordar la sarcopenia de forma integral. El crecimiento del mercado se ve impulsado por el efecto combinado del énfasis en la atención preventiva, la accesibilidad a la atención médica y la concienciación de los pacientes.
Perspectivas del mercado del tratamiento de la sarcopenia en el Reino Unido
Se prevé que el mercado británico de tratamiento de la sarcopenia crezca a una tasa de crecimiento anual compuesta (TCAC) considerable durante el período de pronóstico, impulsado por la creciente concienciación sobre el deterioro muscular relacionado con la edad y los programas de atención médica preventiva. Los profesionales sanitarios recomiendan suplementos nutricionales, fisioterapia y medicamentos para la intervención temprana. Las iniciativas gubernamentales que promueven la salud de las personas mayores y las prácticas de envejecimiento saludable impulsan una mayor adopción. La integración de la salud digital, que incluye la monitorización remota y la telemedicina, mejora la participación del paciente y la adherencia al tratamiento. La adopción de programas de gestión de la sarcopenia en centros residenciales y clínicos está en constante expansión. En general, el énfasis del Reino Unido en la atención preventiva, la independencia funcional y las intervenciones centradas en el paciente está impulsando el crecimiento del mercado.
Cuota de mercado del tratamiento de la sarcopenia en Europa
La industria europea del tratamiento de la sarcopenia está liderada principalmente por empresas bien establecidas, entre las que se incluyen:
- Biophytis (Francia)
- Novartis AG (Suiza)
- Sanofi (Francia)
- Bayer AG (Alemania)
- UCB SA (Bélgica)
- Teva Pharmaceutical Industries Ltd. (Israel)
- H. Lundbeck A/S (Dinamarca)
- Galápagos NV (Bélgica)
- Ipsen SA (Francia)
- Almirall SA (España)
- Corporación Orion (Finlandia)
- Biocryst Pharmaceuticals, Inc. (EE. UU.)
- Haplogen Pharmaceuticals AG (Suiza)
- Medtronic (Irlanda)
- AbbVie Inc. (EE. UU.)
- Pfizer Inc. (EE. UU.)
- Lilly USA, LLC (EE. UU.)
- Bristol-Myers Squibb Company (EE. UU.)
- AstraZeneca (Reino Unido)
¿Cuáles son los desarrollos recientes en el mercado europeo de tratamiento de la sarcopenia?
- En septiembre de 2025, Biophytis presentó su estrategia de ensayo de fase 2 para Sarconeos (BIO101), dirigido a la sarcopenia relacionada con la obesidad. El ensayo se llevará a cabo en Europa y Brasil, con el objetivo de evaluar la eficacia de BIO101 para mejorar la fuerza y la función muscular en pacientes obesos. Esta expansión hacia la sarcopenia relacionada con la obesidad subraya la versatilidad de BIO101 como posible tratamiento para múltiples afecciones relacionadas con la sarcopenia.
- En agosto de 2025, Biophytis anunció la aprobación de la Agencia Europea de Medicamentos (EMA) y de las autoridades reguladoras belgas para iniciar la Parte I de su ensayo clínico de fase 3 con Sarconeos (BIO101), un fármaco candidato para la sarcopenia. Esta aprobación marca un paso significativo en el desarrollo de tratamientos farmacológicos para la sarcopenia en Europa.
- En junio de 2025, Biophytis anunció el inicio del ensayo clínico de fase 3 SARA para Sarconeos (BIO101), un fármaco candidato para el tratamiento de la sarcopenia en adultos mayores. Este ensayo fundamental se lleva a cabo en varios países europeos y está diseñado para evaluar la eficacia y seguridad de Sarconeos para mejorar el rendimiento físico y la fuerza muscular en pacientes sarcopénicos. El resultado de este ensayo podría allanar el camino para el primer tratamiento farmacológico para la sarcopenia en Europa.
- En abril de 2025, una iniciativa europea liderada por el grupo COMET (Medidas de Resultados Básicas en Ensayos de Efectividad) desarrolló un Conjunto de Resultados Básicos (COS) para la sarcopenia. Este COS busca estandarizar los resultados medidos en ensayos clínicos y en la práctica clínica habitual, garantizando la coherencia y la comparabilidad entre estudios. El establecimiento de este COS representa un paso significativo para mejorar la calidad de la evidencia y las estrategias de tratamiento para la sarcopenia en Europa.
- En agosto de 2024, TNF Pharmaceuticals anunció planes para lanzar un ensayo clínico de fase 2b sobre la eficacia de la isomiosamina en la sarcopenia y la fragilidad a principios del primer trimestre de 2025. Este ensayo tiene como objetivo explorar más a fondo la eficacia del fármaco en la sarcopenia/fragilidad, basándose en resultados positivos estadísticamente significativos de un estudio clínico de fase 2 anterior.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.